Impact of access to imaging technologies on scientific achievements

Baubak Bajoghli, who did his postdoc at EMBL Heidelberg between 2012-2017 and is currently the Director of Austrian Bioimaging/CMI, discusses his passion for imaging and his work straddling basic and applied research in biology
Baubak Bajoghli’s interest in microscopy began at an early age and helped him keep expanding his horizons in biological research. In a career spanning both fundamental and translational research, Bajoghli has kept his passion for imaging alive and has recently taken up a role as the Director of Austrian Bioimaging/CMI, where he works to improve access to advanced microscopy infrastructure for researchers working across the country.
We caught up with Bajoghli about his research on cancer-linked immune cells, his key takeaways from his time at EMBL, and the importance of improving access to research infrastructures across Europe.
What triggered the start of your journey in science?
Well, my interest in microscopy began when I was 14 and I visited a medical laboratory for diagnostics for a high school project. My father bought me a microscope, and I had a lot of fun counting all types of white blood cells, including neutrophils and lymphocytes, in patients’ blood smears and comparing my results with those of the laboratory staff. One thing led to another, and my endless curiosity developed into a passion for science.
Can you tell us about your work at EMBL, and how it has influenced your journey as a researcher?
It has consistently influenced my path over the last 20 years. It began when I did an internship during my undergraduate years at Jochen Wittbrodt’s lab in the Developmental Biology Unit, where I learned about using medaka fish as a model organism and transgenesis methods. After a month, I returned to Vienna with 300 medaka eggs in my baggage and, with help from Jochen, my former supervisor, Thomas Czerny, we set up Austria’s first medaka research facility in 2002.
After my post-doctoral fellowship at the Max-Planck Institute of Immunobiology, I became fascinated by the development of T-cells, which belong to our adaptive immune system. The process involves two migratory journeys. First, T-cell progenitors originating from the hematopoietic tissue in the bone marrow must migrate through the body and find the thymus organ. Second, within the thymus, these progenitors have to follow a precise migratory path within different microenvironments, to develop as naive T-cells before they leave the organ.
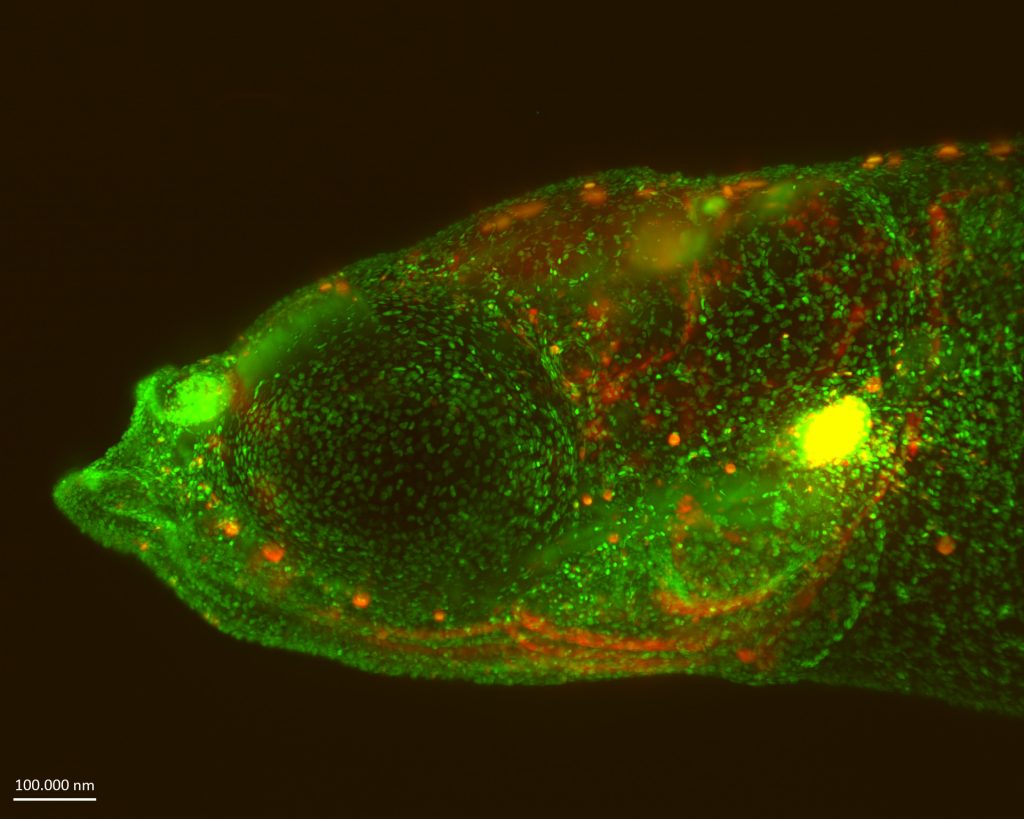
At that time, our knowledge relied mostly on histological sections, and how developing T-cells sense different environments and control their migratory behaviour was less understood. Being skilled in generating transgenic medaka fish, I decided to employ live imaging of the thymus in this species, because imaging of the mouse thymus is technically not possible.
From my time as an intern, I knew that EMBL’s Advanced Light Microscopy Facility (ALMF) was the right place to test this feasibility. In 2012, I joined Maria Leptin’s lab as an EIPOD fellow to pursue my scientific dream and visualised the migratory behaviour of all developing T-cells within an organism using different imaging technologies. The data we generated over five years at EMBL became the foundation of many studies when I became a principal investigator (PI) at the University Hospital Tübingen.
Can you tell us a bit more about your research on immune cells and cancer at the University Hospital Tübingen?
My team focused on two different research topics. First, we continued our work on the T-cell development that began at EMBL. We extracted quantitative data from our in vivo imaging experiments and, for the first time, developed a virtual thymus organ in collaboration with Erika Tsingos. By combining cell-based computer modelling and in vivo manipulation of the thymic niche, we uncovered how the interaction between cell location, signals from the thymic niche, and the timing of gene expression affects the fate decision of progenitor cells in the thymus.
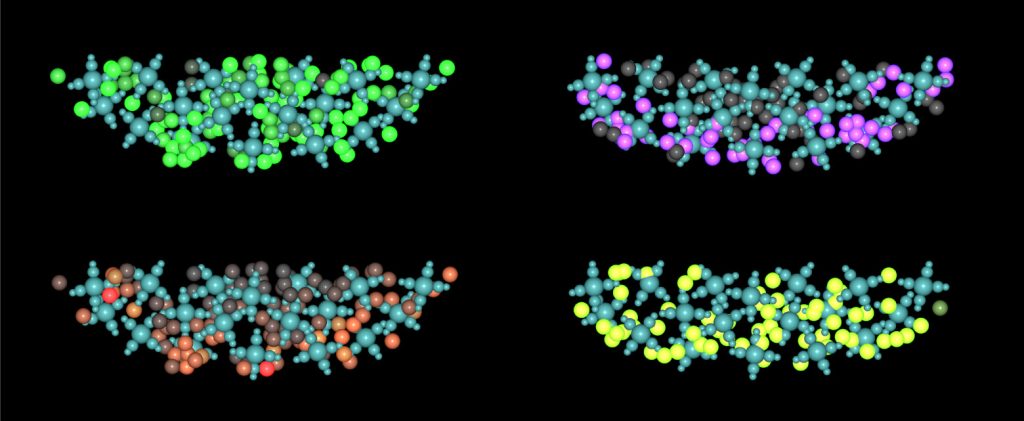
Furthermore, we enhanced our virtual thymus model to investigate the causes of T-cell lymphoblastic leukaemia (T-ALL), which is still a long-standing mystery. Through computational simulations of over 300 scenarios, we pinpointed the minimum requirements for the clonal expansion of a single developing T-cell – a process wherein immune cells divide quickly to give rise to many clones – which we confirmed experimentally. The strategy that we have developed not only provided us with a rapid and comprehensive overview of the outcomes in all scenarios but also helped in reducing the need for animal experiments, thus implementing the principles of the 3Rs (reduce, reuse, recycle) in science. Currently, we are finalising the data analysis and preparing to draft a research manuscript outlining our findings.
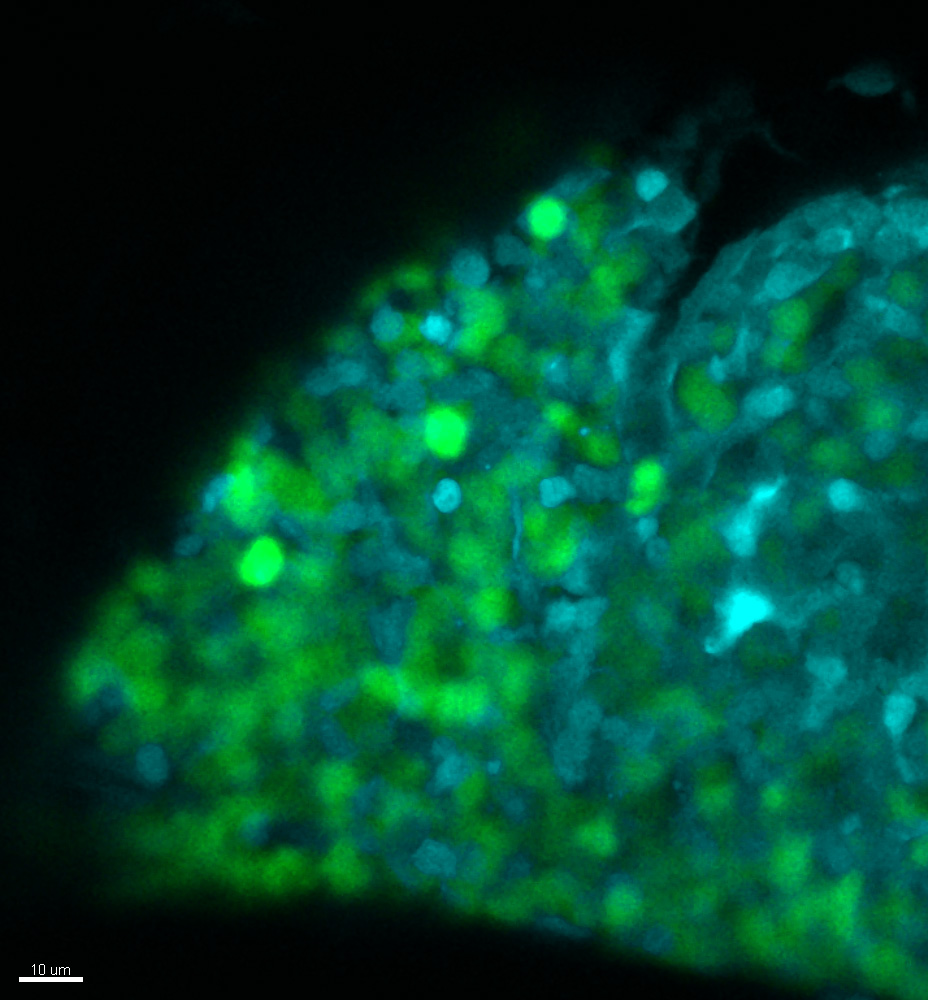
The focus of the translational oncology division, where I was hosted, was to find new ways to treat congenital neutropenia, a rare blood disorder that affects patients, mostly young children, causing extremely low levels of neutrophils (a type of white blood cell) in their blood. Consequently, these patients are highly susceptible to bacterial infections, and today, their only treatment option is a daily injection of a cytokine for their entire lives, which unfortunately increases their risk of developing leukaemia by 20%. Therefore, my team strongly contributed to collaborative projects which helped to better understand the underlying mechanisms. We developed various zebrafish models for congenital neutropenia. Also, we established new patient-derived xenotransplantation models (systems where patients’ samples are implanted into a model organism like zebrafish or mice) to test the effectiveness of various small molecules against the proliferation of leukemic cells.
Your work has spanned both basic and translational aspects. Could you tell us a bit about the connections between the two and your experience working across both worlds?
After 15 years of working in basic research, moving to translational research was a big transition in my scientific career. You need to adjust your mindset because, as you said, these are two different worlds. Before, I was used to asking fundamental questions about how biological processes work. When I became a PI in the translational oncology division, my main focus shifted towards developing new tools for preventing or treating diseases.
I quickly realised that success in applied research relies heavily on a solid foundation of basic research. Even something as seemingly distant as understanding the evolution of genes can be incredibly helpful in designing new tools for fighting human diseases.
Take the gene called ELANE, for example. About 45% of patients with congenital neutropenia have missense mutations in this gene. When I explored the evolution of this gene and used the ENSEMBL database, I noticed that ELANE is a product of tandem gene duplication that occurred in the mammalian lineage, and lower vertebrates don’t have this gene.
Because I knew from previous studies published by other colleagues that the development of neutrophils is evolutionarily conserved across vertebrates, I made the case to medical scientists that if fish can develop neutrophils without ELANE, then maybe this gene isn’t as crucial for human neutrophil development as we once thought. And it turned out to be true. When they knocked out the dysfunctional ELANE gene in induced-pluripotent stem (iPS) cells derived from patients, they were able to develop as neutrophils normally.
Now, ongoing work at the University Hospital Tübingen is focused on establishing this as a new therapeutic approach for these patients. It’s a great example of how basic research can lead to practical solutions in the field of medicine. Unfortunately, basic research often doesn’t get the recognition it deserves from funding bodies and the public, but it needs to be properly appreciated.
You are currently the Director of Austrian Bioimaging/CMI. Can you tell us what motivated this transition?
Again, my passion for imaging and my personal experiences as a researcher played a significant role in my decision to make a huge transition in my career path, this time from a researcher to a research infrastructure provider. When I started my own lab, my host institute had promised me access to the necessary microscopes for my fully funded thymus project. However, the reality didn’t match the promise, and I had to invest a lot of time into figuring out which institutes had the right microscopes and whether my team could use them.
It was frustrating because we could not use the imaging facilities of other institutes due to legal policies. In the end, with Maria Leptin’s support, we could fortunately use the Advanced Light Microscopy Facility (ALMF) at EMBL. For almost two years, multiple times a month, I drove 180 kilometres from Tübingen to Heidelberg in the morning, performed imaging at the ALMF, and then drove back home in the afternoon.
It was challenging, but I was grateful because, without access to the right microscope, we could not have answered a 30-year-old question about how some species, despite using the same molecular mechanism and the same progenitor cells, develop a higher frequency of certain T-cell sublineages compared to others. In the long term, this discovery might help in the development of new therapeutic applications, as some T-cell sublineages have antitumour functions, yet their proportion in the human body is less than 1%.
My own experiences have made me acutely aware that many scientists have outstanding research projects but struggle to conduct experiments properly due to a lack of equipment, expertise, or resources at their host institutions. On the other hand, I understand that it’s not economically feasible for institutions to invest in every expensive instrument their employees might temporarily need. In my opinion, the most effective solution to this problem is taking advantage of pan-European consortia of research infrastructures that offer access to cutting-edge technologies for all researchers, regardless of their institutional affiliations.
Can you tell us a bit more about Austrian BioImaging/CMI and how consortia like these can help researchers?
Austrian BioImaging/CMI is one such consortium at the national level, consisting of eight Austrian universities and leading research institutions. We enable researchers to access over 40 imaging technologies for biological and preclinical research, and as a node, we strongly cooperate with Euro-BioImaging, which is a European Research Infrastructure Consortium (ERIC) member.

During my 18 months in office, I’ve been committed to ensuring that researchers don’t face the same challenges I did. To me, open access to research infrastructures should be a pillar of open science in European policy. I don’t tire of communicating with researchers, heads of universities and funding bodies, and Austrian policymakers to raise their awareness about the key role of state-of-the-art research infrastructures in generating knowledge breakthroughs and new discoveries, and why their sustainability is so important. I’m appears that the European Commission also recognises their importance.
Currently, there are several Horizon Europe programs that financially support researchers’ access to high-quality resources, including biological and biomedical imaging technologies. I really recommend that researchers always keep themselves well-informed so that they can make the maximum use of the available resources for their projects. Since there are numerous options available, Austrian Bioimaging/CMI and Euro-Bioimaging also provide consulting services for researchers.
In your opinion, how do initiatives like Euro-Bioimaging and Austrian Bioimaging influence the way biological research is done in European countries (and globally)?
To strengthen our competitiveness in global research, specific attention should be given to national and European research infrastructures consortia. To me, their socio-economic impact is unquestionably high. In today’s rapidly evolving technological landscape, access to cutting-edge technologies and services is crucial for performing outstanding research. However, building and maintaining cutting-edge research infrastructures can be expensive and by sharing these costs, individual institutes and countries can reduce the financial burden, making research more economically sustainable in Europe.
Additionally, European research infrastructure consortia such as Euro-BioImaging have the potential to act as incubators for innovation and technology transfer. In Austrian BioImaging/CMI, 40% of our technology units consist of research groups specialising in various imaging modalities, and developing tools for biological research or medical diagnostics.
It’s important to note that we are just one of 35 nodes within Euro-BioImaging. So, there is a huge potential for cooperation between 173 imaging facilities and research groups from 16 countries and EMBL associated with Euro-BioImaging, as well as with the private sector, to drive innovation and address future research needs. Solutions for open bioimaging data, common standards and best practices for biological and medical imaging can only be achieved at the pan-European level.
Last but not least, research infrastructure consortia must cooperate to enhance their visibility because we can only attract top talent when we can provide access to cutting-edge research infrastructures in Europe, which in the long run, will also bring financial benefits.
What is one piece of advice you would give to young researchers just starting their scientific journeys?
Find something that is your passion and dedicate your life to it.