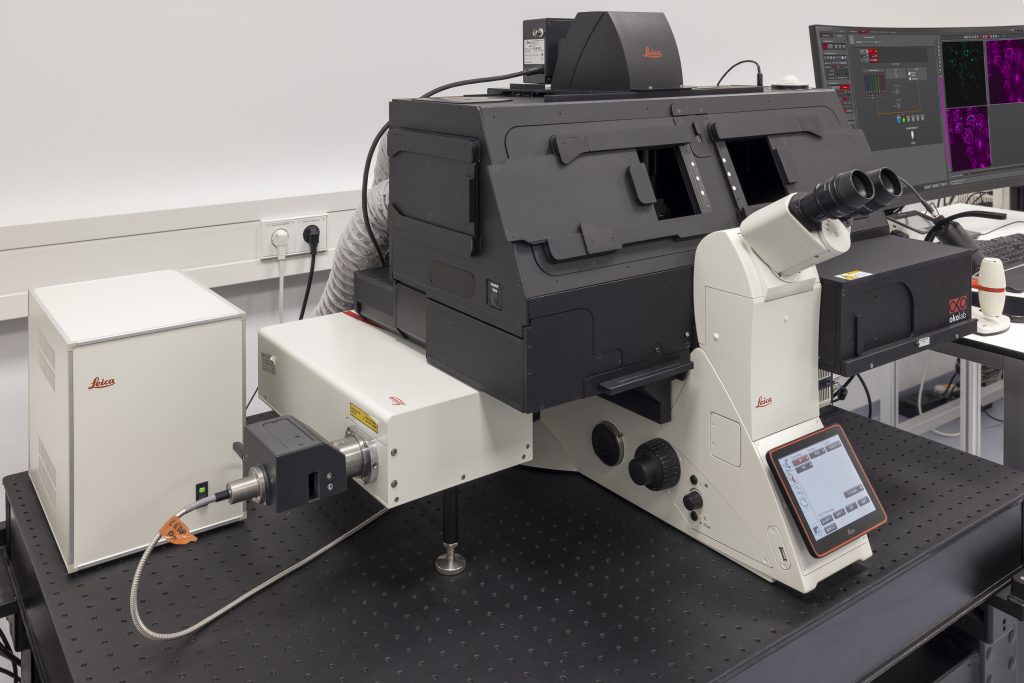
STELLARIS 8 STED Falcon
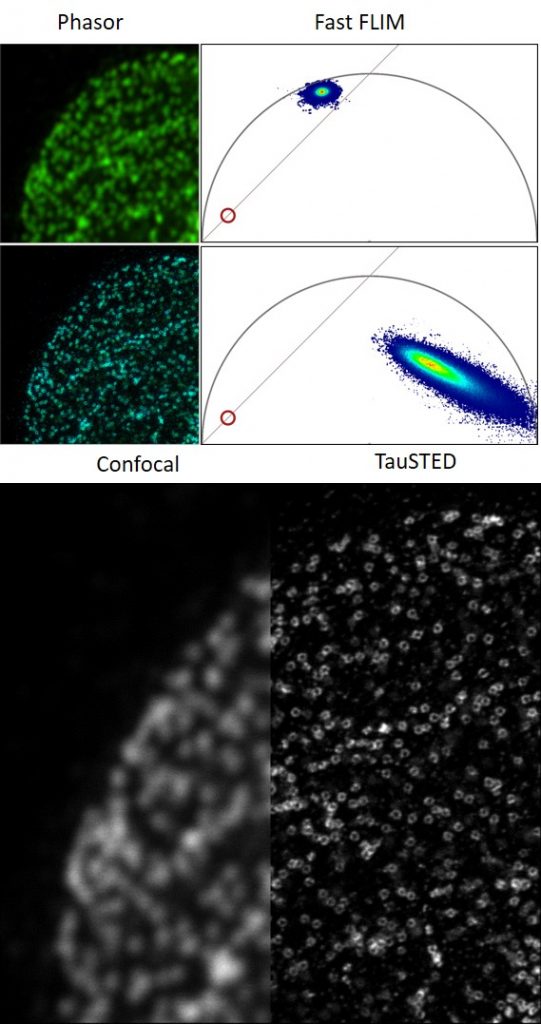
STELLARIS 8 STED with TauSTED from Leica Microsystems allows the study of multiple dynamic events simultaneously and investigation of molecular relationships and mechanisms within the cellular context.
FALCON (FAst Lifetime CONtrast) includes phasors for quantitative FLIM analysis. It is a fully integrated solution for fluorescence lifetime imaging (FLIM) and enables video-rate image acquisition for rapid kinetic studies in live cells.
Features
- Resolving molecular relationships in specimens
- Multiple simultaneous events can be studied at the nanoscale
- 2D and 3D STED imaging
- Significantly reduced light doses due to TauSTED
- Advanced STED-FLIM options with STED and FALCON (full FLIM quantitative tools, phasor FLIM, species separation based on phasor analysis)
- Advanced STED-FCS options
- Fast validation of results on a single platform, from confocal to super-resolution LIGHTNING and STED
- With FALCON it is possible to:
- follow fast molecular interactions via FLIM-FRET (Förster resonance energy transfer)
- use biosensors to detect microenvironmental changes, such as pH or ion concentration
- apply lifetime contrast to separate multiple fluorophores
- Analysis using phasor FLIM provides a 2D visualisation of lifetime components. With FLIM phasors you can follow microenvironmental changes, select components to multiplex signal (phasor separation), and determine FRET efficiency
Specifications
- Five spectrally tunable Power HyD sensitive photon counting detectors (2 HyD S, 2 HyD X, 1 HyD R)
- Confocal: tunable White Light Lasers (WLL) 440-790 nm, 405 nm STED depletion: 592 nm, 660 nm, 775 nm
- 8kHz Resonant Scanner
- Total system dead-time: 1.5 ns
- TauSTED: Tunable resolution based on lifetime (depending on sample and fluorophore: <30 nm (lateral) and <100 nm (axial). Automatic lifetime-based background suppression algorithm. Light dose reduction (WLL excitation) for all STED lines (592, 660, 775 nm). Available for 2D and 3D STED in live and in fixed specimens, also for multicolor applications. Automated workflow integrated in the LAS X software
- The STED WHITE glycerol and water objective lenses with motCORR technology provide adaptive optical correction for aberrations introduced by sample inhomogeneities and refractive-index mismatch. The STED WHITE objective lenses provide a working distance of 300 µm:
- HC PL APO 86x/1.20 W motCORR STED WHITE
- HC PL APO 93x/1.30 GLYC motCORR STED WHITE
- HC PL APO 100x/1.40 OIL STED WHITE

MICA
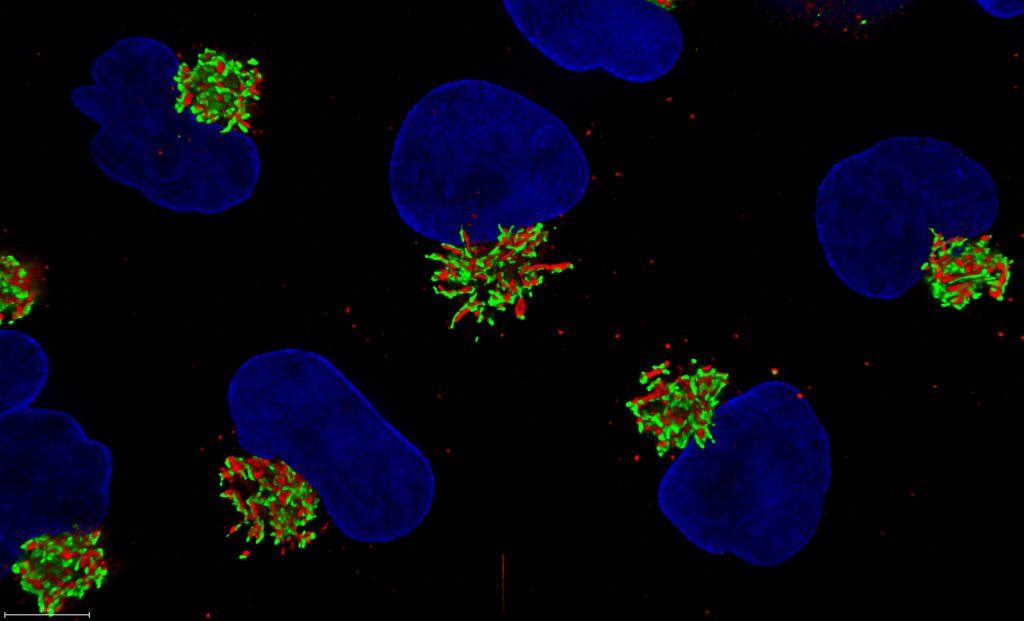
Mica from Leica Microsystems is a highly automated microscope that unites widefield and confocal imaging. The user interface automates sample-finding, parameter setting and image focus, reducing imaging time. Through intelligent automation, all opto-digital components are fully motorized and automated, reducing the number of setup steps needed to generate images. The Mica has full environmental control capabilities.
Features
- Simultaneously visualize four colors in widefield or confocal, rapidly switching between modalities
- FluoSync™ spectral unmixing
- Integrated incubation system
- On-board image analysis features (e.g pixel classifier and identification of parameters required for segmentation)
Specifications
- Integrated modulation contrast (IMC) and brightfield transmitted light imaging in RGB or gray scale mode
- Incident fluorescence illumination: LED 365 nm, 470 nm, 555 nm, 625 nm
- 4 simultaneous widefield detection channels with FluoSync spectral unmixing
- Confocal illumination: Laser diode 405 nm, 488 nm, 561 nm, 638 nm
- 4 simultaneous confocal detection channels (HyD FS) with FluoSync spectral unmixing
- Environmental control: Temperature (room temperature +3 °C to 45 °C), CO2 (0 – 10 %), humidity
- Closed loop water dispenser for objective immersion. Water immersion for one objective is feedback controlled and does not require any user interaction
- THUNDER Methods: Instant Computational Clearing (ICC), Small Volume Computational Clearing (SVCC), Large Volume Computational Clearing (LVCC)
LIGHTNING Methods Basic, LIGHTNING Expert
THUNDER Imager Live Cell
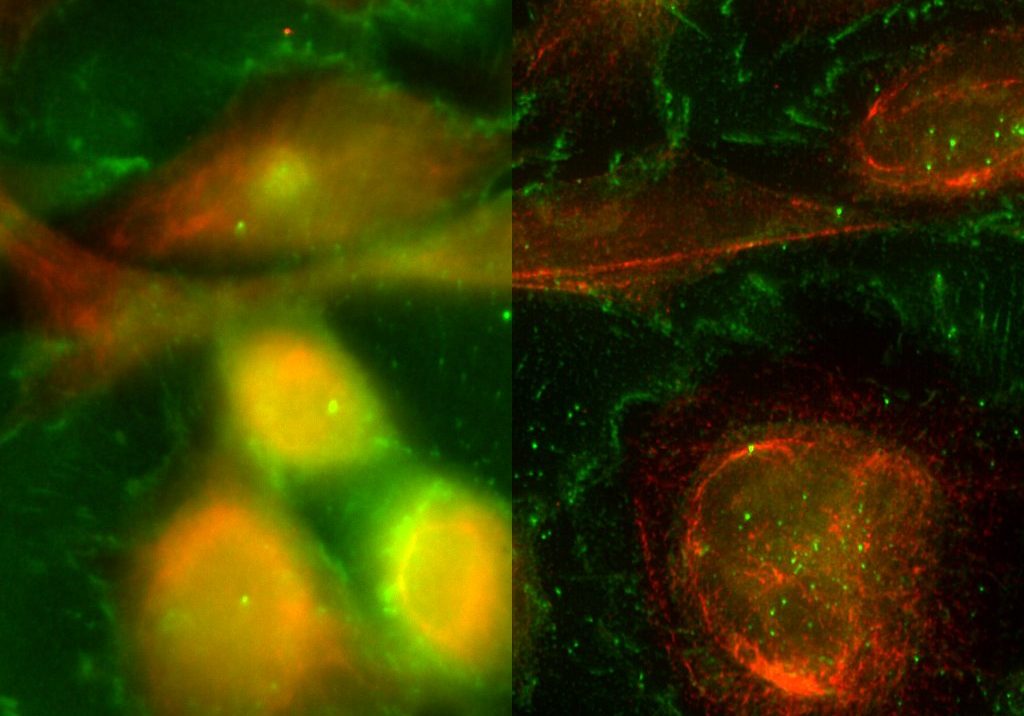
THUNDER is an opto-digital technology from Leica Microsystems that uses the Computational Clearing method for haze removal to generate high-resolution and high-contrast images.
A THUNDER Imager Live Cell provides a complete microscopy imaging system for minimally invasive and precise live-cell imaging. High-speed and/or long term examination of specimens, e.g. cell cultures and organoids, can be performed at low phototoxicity and photobleaching.
Features
- sCMOS technology
- Efficient removal of out-of-focus blur in real time with Computational Clearing
- Automation of the imaging and analysis workflow within the LAS X software
- Further processing and reconstruction using the Aivia software platform
Specifications
- Based on the fully motorised, high-end, inverted research microscope DMi8
- High-speed positioning with the Quantum stage and Synapse real-time controller
- High-speed illumination with a multi-line LED light source
- Fast switching external filter wheel
- Adaptive Focus Control (AFC) with closed loop focus
- Climate chamber ensures optimal physiological conditions for living cells
THUNDER Imager Live Cell is part of the Coral Life live cell CLEM workflow consisting of the THUNDER Imager Live Cell, the EM ICE high pressure freezer and the EM AFS2 for automatic freeze substitution.

DMi8 S with TIRF module
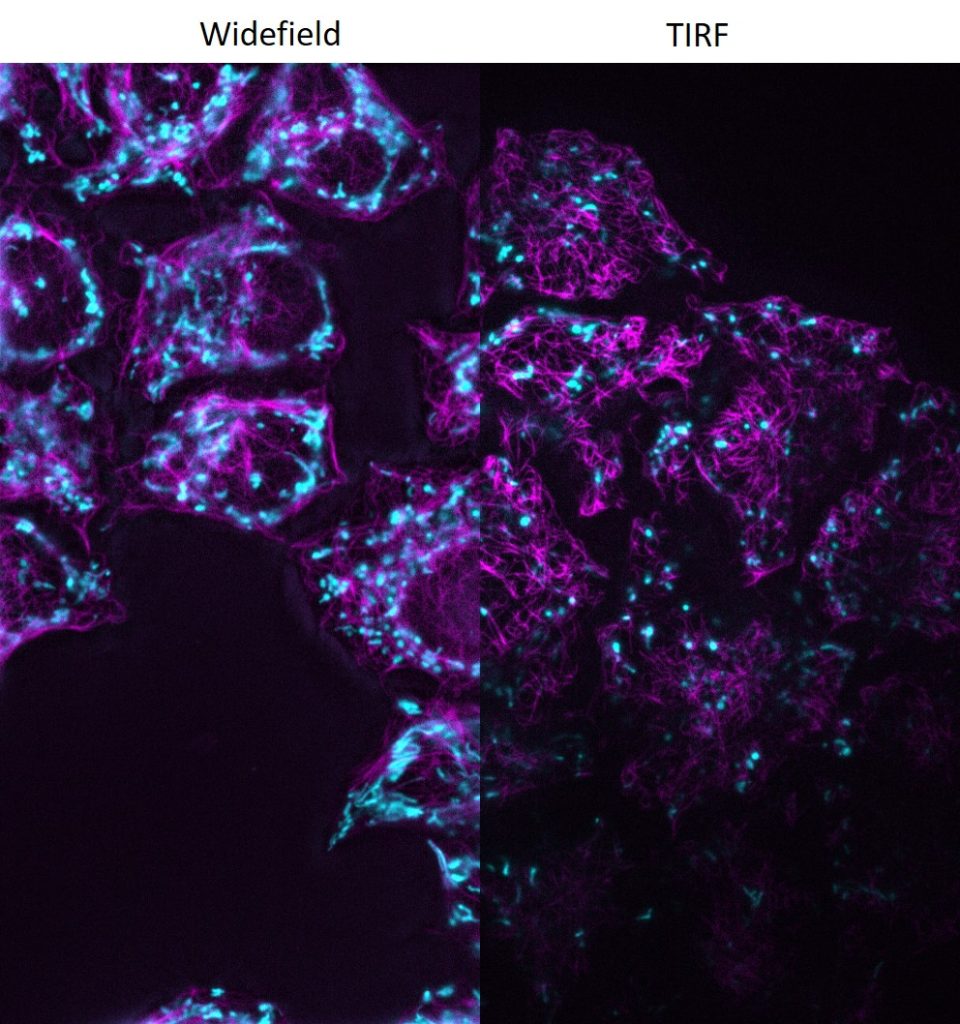
DMi8 S from Leica Microsystems is a live cell imaging system to execute advanced experiments with imaging modalities like TIRF. For dynamic processes at the cell surface TIRF (Total Internal Reflection Fluorescence) microscopy is the method of choice to visualise single molecules with super-resolution by maximising the fluorescent signal- to-noise-ratio.
Features
- Infinity TIRF module with azimuth shifting capability to optimise the illumination field.
- LAS X Navigator software to create quick overviews of the sample and identify the important details instantly. High resolution image acquisition can be set up using templates for slides, dishes, and multi-well plates.
Specifications
- Fully motorised DMi8 inverted research microscope with integrated real-time controller to operate the system with microsecond precision
- Adaptive Focus Control (AFC)
- Equipped with a high-end sCMOS camera
Coming soon: OPM
More information will follow soon…