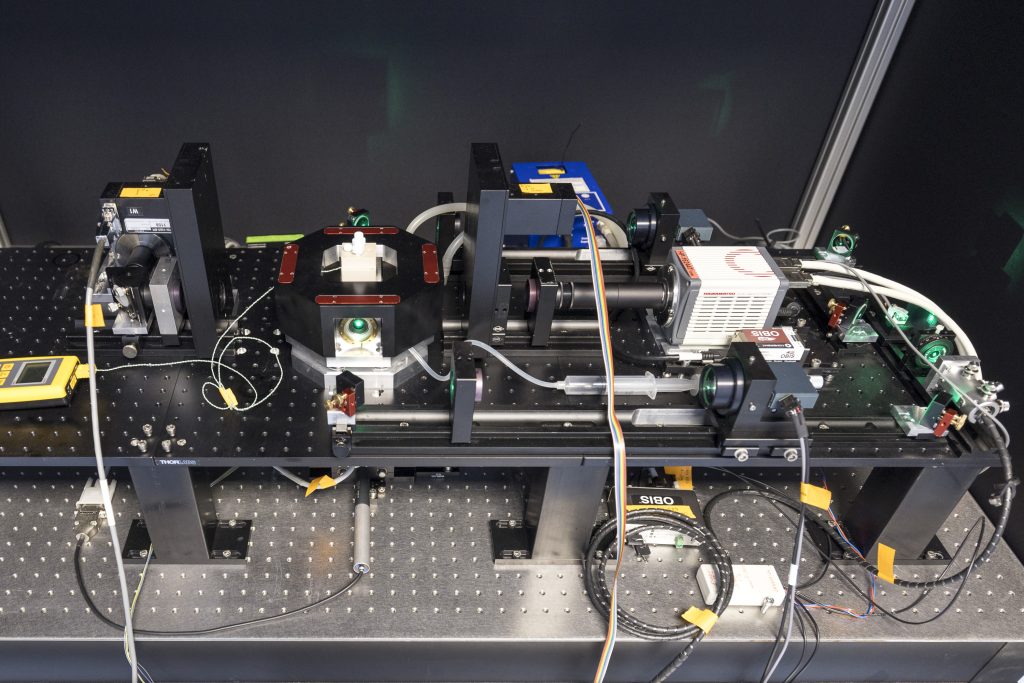
MultiView SPIM
The MultiView-SPIM or MuVi-SPIM (selective plane illumination microscope) is an advanced light-sheet fluorescence microscope utilising multiple orthogonal optical paths for in vivo imaging of small embryos, organisms and cell cultures. The MuVi-SPIM available at the EMBL IC has originally been developed by the lab of Lars Hufnagel at EMBL and further developed by the lab of Robert Prevedel at EMBL. Owing to its plane wise illumination confinement and fast imaging on multiple cameras, the MultiView-SPIM is able to capture developmental dynamics with minimal photodamage to the living subjects.
Features
- Multi-view reconstruction via fusion of multiple camera views
- Environmental control (Temperatures, CO2 etc.)
- Variable magnification 22 – 33x (600 – 400 um field of view)
- Sub-micron resolution for superficial imaging, performance at depth dependent on sample optical properties.
- Imaging typically at 50 – 100 frames per second, volume rate typically 0.1 – 1 volume per second
- Multi-color imaging achieved sequentially with fast filter wheels
- Live samples can be imaged over several days
Specifications
- Light sheets available at 488, 515, 594 nm (and 685, 808 nm on request)
- Light sheets generated by beam scanning and combined with camera-based confocal line detection to reject blur and enhance contrast
- Motorised x,y,z and rotation stages
- Sample mounting in refractive index matching tubes (FEP n = 1.33) or extruded in gelated column from glass capillaries
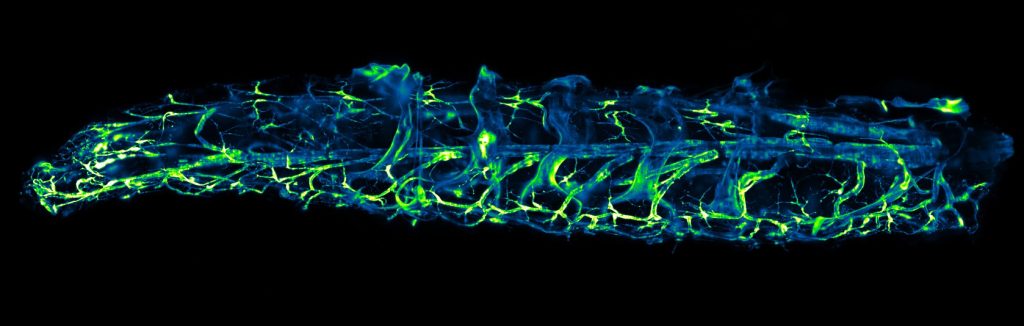
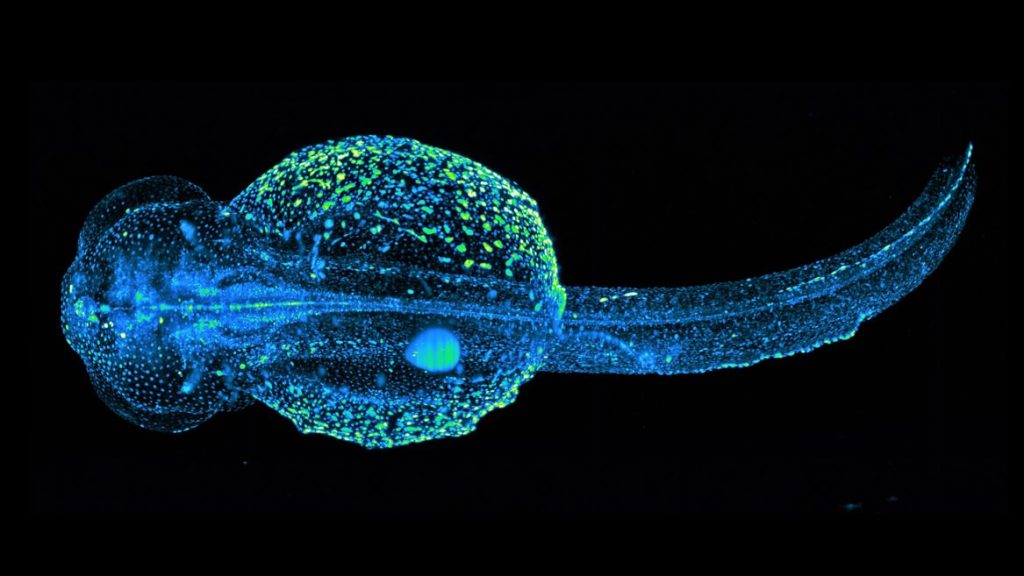
STELLARIS 8 DIVE Falcon
STELLARIS 8 DIVE with the 4Tune detection system is a spectrally tunable multiphoton microscope from Leica Microsystems. Four non-descanned spectrally flexible channels provide multicolour multiphoton imaging at > 1 mm depth.
Lifetime-based information can be directly obtained in addition to distinguish spectrally similar fluorophores, separate second harmonic (SHG) signals from fluorescence or visualise the metabolic state of cells. Lifetime analysis and quantification can be done FALCON.
Features:
- 4 spectrally tunable NDD detectors in the visible range (380 – 800 nm)
- Qualitative and semi-quantitative lifetime-based information with TauSense
- Fully quantitative FLIM analysis with FALCON, including phasors
Specifications:
- Laser lines: IR: tunable 680 – 1080 nm and 680 – 1300 nm, fixed 1040 nm; Confocal: White Light Lasers (WLL) 440 – 790 nm, 405 nm, and 488 nm
- Four NDD channels equipped with Power HyD X (tunable from 380 – 800 nm), five spectrally tunable internal counting detectors (3 HyD S, 1 HyD X, and 1 HyD R)
- Upright confocal fixed-stage (DM6 CFS) stand furnished with Scientifica scanning stage

THUNDER Imager 3D Tissue
The THUNDER Imager Tissue from Leica Microsystems allows real-time fluorescence imaging of 3D tissue sections typically used for neuroscience and histology research. It also enables the acquisition of rich, detailed images of thick tissues. These images are free of haze from out-of-focus blur because of Computational Clearing for haze removal. Structures like axons and dendrites of neurons in a brain slice can be imaged.
Features
- Rapidly acquisition of blur-free images even deep within thick sections
- Get fast overviews of whole tissue sections
- Imaging and analysis workflow within the LAS X operation software
- Processing and reconstruction of complex images with the Aivia software platform
Specifications
- Based on a fully automated upright research microscope for the acquisition of multi-color 3D images
- sCMOS camera system
- Software creates blur-free large overviews of the entire tissue specimen
- Precise motorised z-focus drive to capture images in the z-direction and visualise them with the 3D Viewer

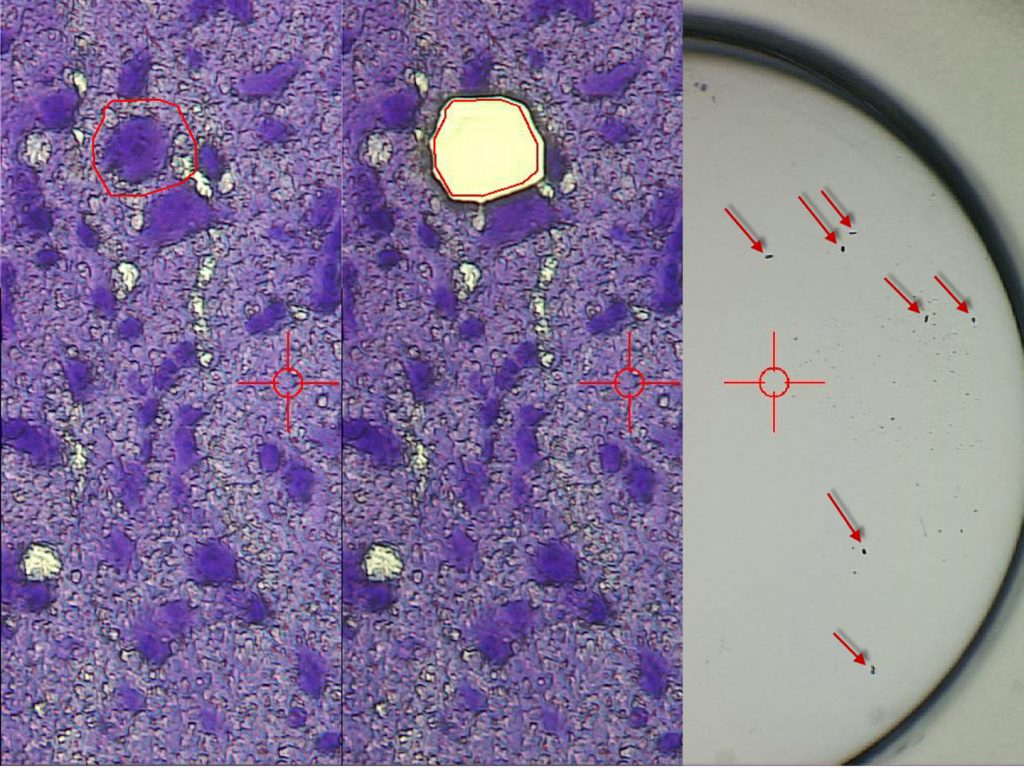
LMD7
The LMD7 laser microdissection microscope from Leica Microsystems enables users to isolate specific microscopic target areas under visual control for downstream molecular biology analysis. Regions of interest (ROI) can be isolated from entire areas of tissue, like cell clusters or tumours, down to single cells or even subcellular structures, such as chromosomes. Live cell dissection for downstream analysis or re-cultivation (cloning) as well as laser manipulation is possible. The LMD7 is equipped with a LMT350 scanning stage which allows collection of dissectates directly into regular PCR tubes or multi-well plates, such as 96-well PCR plates. Downstream analysis is typically used for genomics (DNA), transcriptomics (mRNA, miRNA) by qPCR, Microarray, RNA-seq, next generation sequencing (NGS), proteomics, metabolomics done with Western blots, and mass spectrometry. Lipidomics can be done with laser microdissection.
Features
- Laser movement by optics
- Direct collection into 96-well plates simply by gravity
Specifications
- Wavelength: 349 nm
- Pulse frequency: 10–5,000 Hz
- Pulse length: <4 ns
- Average pulse energy: 120 μJ
- Range of dedicated LMD objectives: 2.5x, 5x, 10x, 20x, 40x, 63x, and 150x