MINFLUX
Minimal Photon Fluxes Microscopy
Open access to cutting-edge electron and light microscopy
We provide researchers from Europe and beyond with a synergistic portfolio of imaging services including cryo-EM, super-resolution and intravital microscopy to enable new ground-breaking research that crosses the scales of biology.

Minimal Photon Fluxes Microscopy
Single Molecule Localisation Microscopy
Stimulated Emission Depletion Microscopy
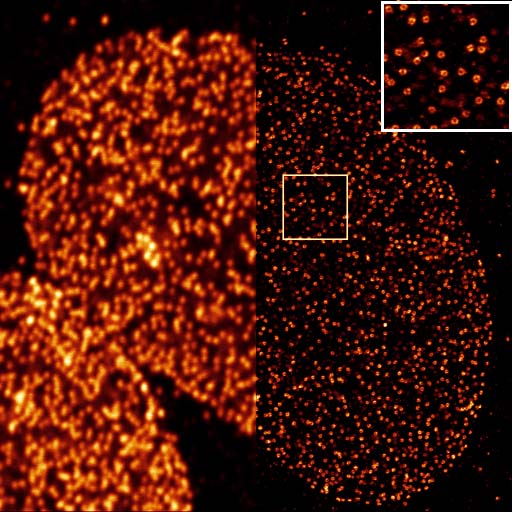
The imaging of minimal photon fluxes combines a coordinate-targeted single point scanning approach with the localization of stochastically activated single fluorophores to provide the currently highest resolving form of super-resolution microscopy.
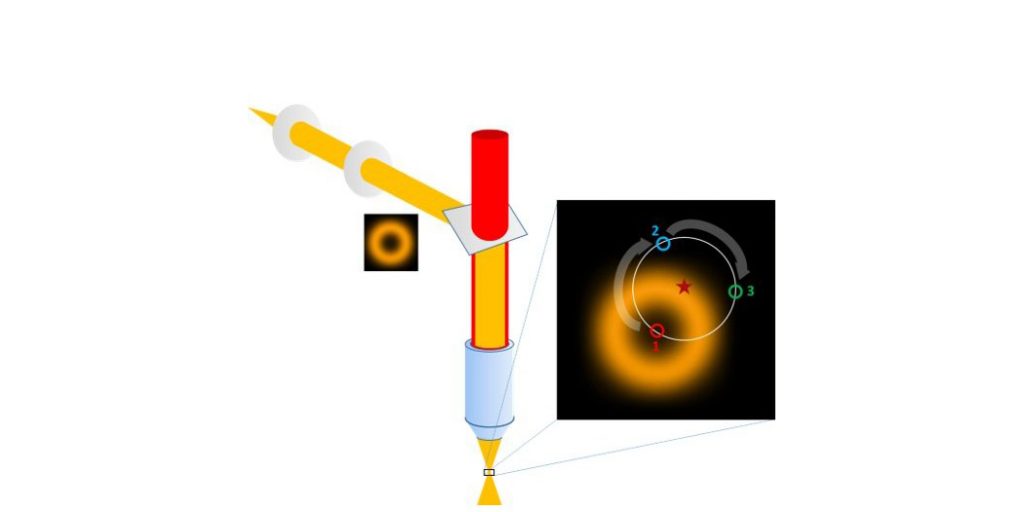
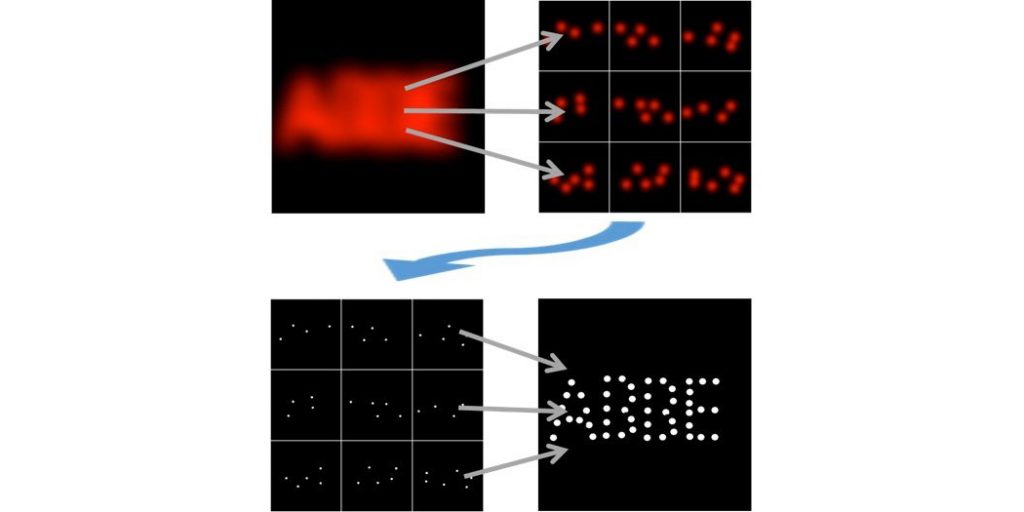
Upon encountering a single point source signal, the exact position of a single fluorophore is interpolated through the photons collected at different positions of a series of patterns with decreasing search ranges that are executed by the probing beam (a concentric illumination ring with a central minimum) to home in on the fluorophore position. The combination of beam targeting with single molecule detection thus allows a localization accuracy in the nanometer range while only requiring a minimal number of photon detections overall. The MINFLUX principle can be applied for highly resolved imaging (stochastically activated fluorophores in a sample are imaged sequentially and integrated into a set of coordinates) as well as for the dynamic tracing of a single fluorescent molecule at unprecedented temporal and spatial resolution.
In SMLM is based on the fact that in diffraction-limited optics the position of a single point (i.e. a single molecule) can be determined with much higher accuracy than the minimal resolvable distance between two points.
Applying a fit for the central maximum of a single point source can thus determine its position with an accuracy that can be an order of magnitude higher than the optical resolution of the imaging system used. This cannot be applied in standard imaging as single point sources cannot be separated from their neighbours while all of them are detected together. In SMLM the label is made sufficiently scarce for the detection of single molecule positions by either the sparse photoconversion of single molecules to an active state (Photoactivation Localisation Microscopy, PALM), by the induction of transient darkstates in a vast majority of the fluorophores in the image field with only randomly distributed single emitters on at any time (Stochastic Optical Reconstruction Microscopy, STORM) or by the transient binding of diluted fluorescent tags to their targets (Points Accumulation for Imaging in Nanoscale Topography, PAINT).
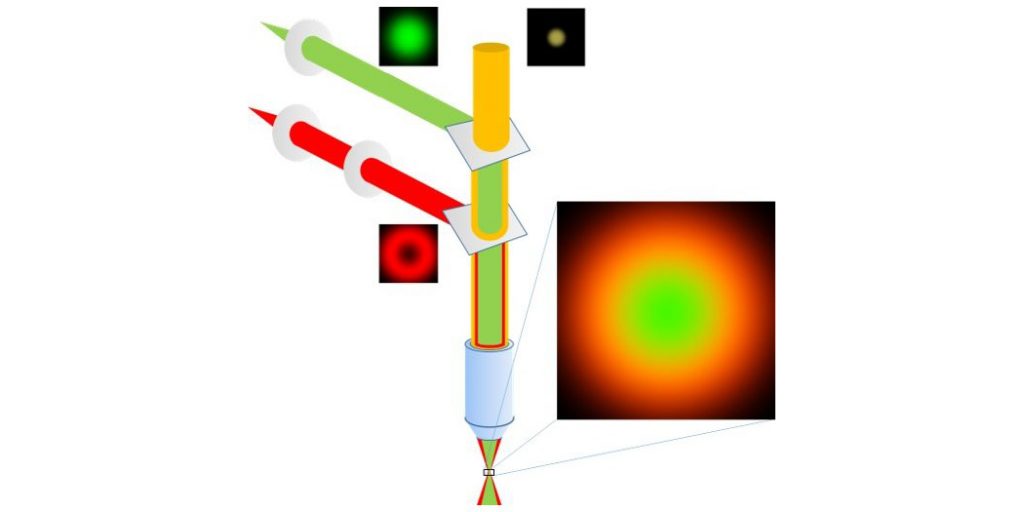
In STED microscopy, an excited fluorescent molecule is induced to return to the ground state through the interaction with a photon of a wavelength inside the fluorophore’s emission spectrum.
This process is called stimulated emission. The combination of a focused excitation beam with a phase-shifted depletion beam in the same position creates a central excitation volume that is surrounded by a concentric ring of depletion light that deactivates fluorophores in its area and thus will reduce the effective fluorescence detection volume. In combination with confocal scanning this allows imaging below the diffraction limit. The resolution improvement is determined by the intensity of the applied depletion and can either be applied directly or be further enhanced by analysing the distribution of shortened fluorescence lifetimes that are caused by the interaction with the depletion beam.
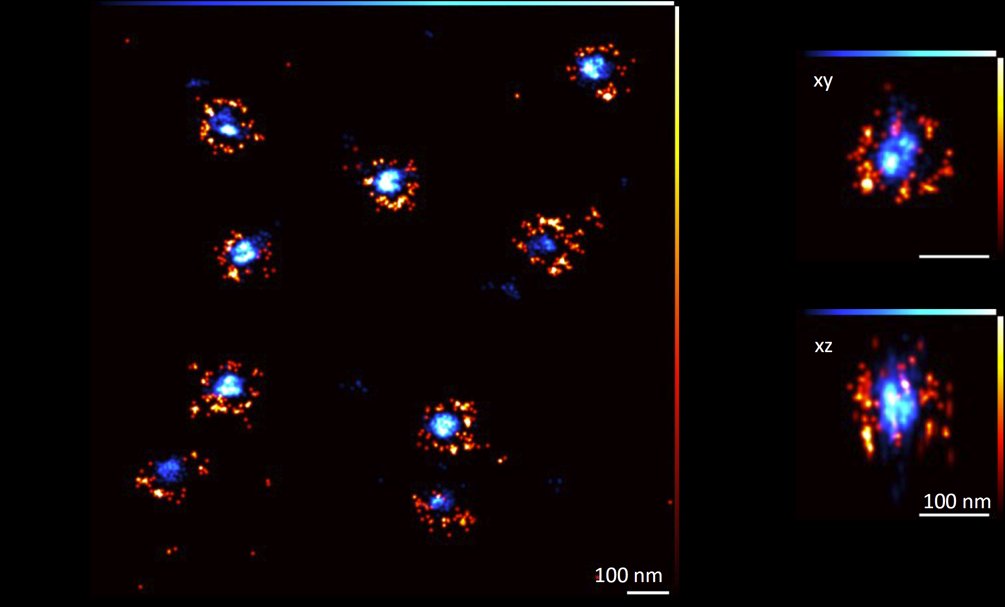
MINFLUX from Abberior Instruments enables imaging of a broad range of biological samples with a spatial resolution in the single nanometer range. This allows to study the structure of large multimolecular protein complexes, such as nuclear pores with unprecedented 3D resolution.
Additionally, MINFLUX tracking allows to study movement of single molecules in living cells with up to 10 kHz sampling rate (one localisation each 100 µs, which is up to 100 x faster than conventional camera-based tracking).
Features:
Specifications:
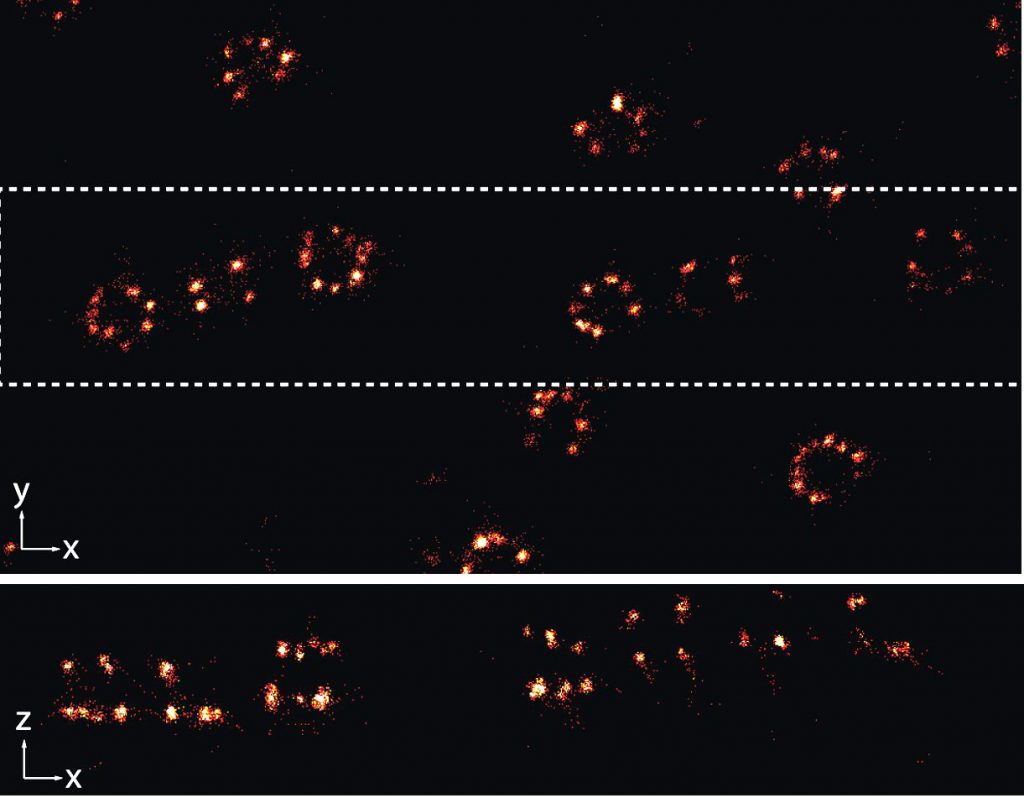

Three-dimensional single-molecule localisation microscopy (3D-SMLM) is an advanced light microscopy methodology based on localisation of sparse single-molecule emitters for computational reconstruction of a super-resolved image. The 3D-SMLM at the EMBL IC has been developed in collaboration with the lab of Jonas Ries at EMBL. At the core of the microscope is an extremely stable inverted microscope. (f)PALM/(d)STORM workflows can be accommodated and additional functionalities will be added dependent on user need (e.g. support for live-cell imaging/PAINT/SOFI and others).
Features
Specifications
STELLARIS 8 STED with TauSTED from Leica Microsystems allows the study of multiple dynamic events simultaneously and investigation of molecular relationships and mechanisms within the cellular context.
FALCON (FAst Lifetime CONtrast) includes phasors for quantitative FLIM analysis. It is a fully integrated solution for fluorescence lifetime imaging (FLIM) and enables video-rate image acquisition for rapid kinetic studies in live cells.
Features
Specifications
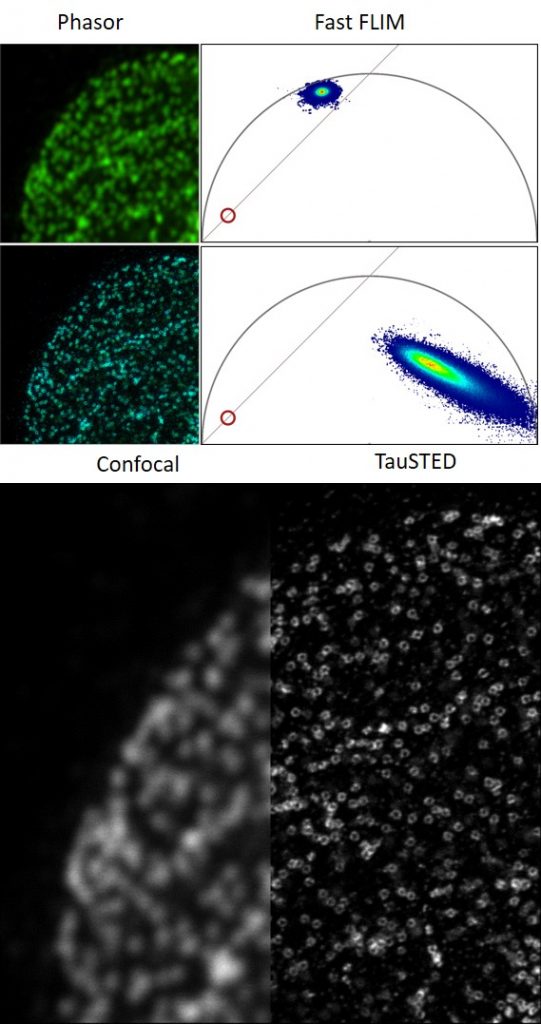

STELLARIS STED equipped with TauSTED from Leica Microsystems allows the study of multiple dynamic events simultaneously and investigation of molecular relationships and mechanisms within the cellular context.
Features
Specifications