Claire Deo
Visiting Group Leader (Outgoing)
ORCID: 0000-0003-0119-3118
EditBuilding next-generation fluorescent tools for biological imaging

Visiting Group Leader (Outgoing)
ORCID: 0000-0003-0119-3118
EditThe fundamental challenge of modern biology is to unravel how biomolecules regulate the complex functions of cells. Fluorescence microscopy has become a powerful tool to decipher the molecular underpinnings of biological systems, as it allows monitoring of biochemical details inside cells with high spatial and temporal resolution. However, the investigation of many intricate cellular processes is only beginning and new discoveries will require more effective fluorescent reporters. Our group aims at developing novel chemical tools to push the frontier of fluorescence imaging and allow interrogation of biological systems at the molecular, cellular and organismal levels.
We design and build innovative probes by combining the superior fluorescence properties of synthetic fluorophores with the specificity of genetically encoded protein scaffolds. This approach, at the interface of synthetic chemistry and protein engineering, has the promise to overcome many limitations of currently available reporters. As tool-builders, we work in close collaboration with microscopists and biologists who implement our tools to ultimately increase our understanding of complex cellular functions.
Our group aims to further explore new mechanisms for the development of bright, modular and specific fluorescent tools for biological imaging. Future research interests include:
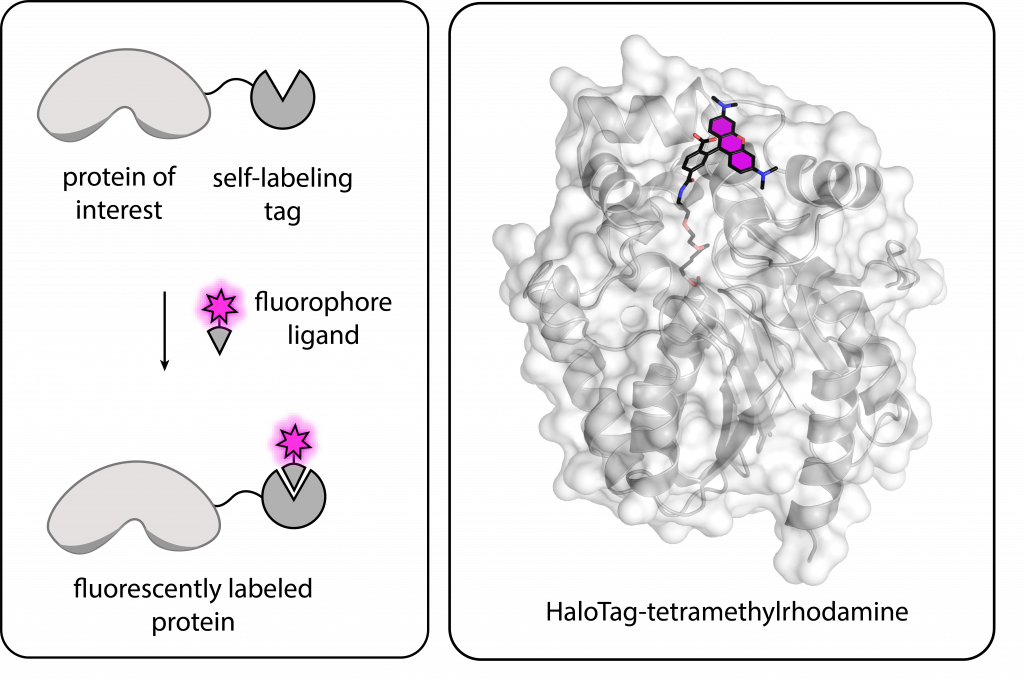
We will develop novel methods for the fast and specific labelling of biomolecules such as proteins and nucleic acids. The goal is to expand the available portfolio of labeling methods to allow simultaneous visualization of multiple biomolecules within cells using small-molecule fluorophores. In particular, we will focus on the design of multicolor fluorogenic markers, which show no fluorescence signal until bound to their target, ensuring low background.
Fluorophores that can be activated with light are key components for super-resolution imaging, enabling the visualization of biomolecular structures below the diffraction limit. We will develop photoswitchable fluorescent reporters compatible with live cell imaging, that are particularly promising for super-resolution microscopy techniques such as single molecule localization microscopy.
Photoacoustic microscopy is an imaging modality which relies on the detection of acoustic waves, following light absorption. In contrast with fluorescence microscopy, this imaging modality allows imaging at centimeter depths. We are interested in developing a novel class of probes for photoacoustic imaging, providing high contrast in vivo, and capable of reporting dynamic changes in small molecules and ions concentration.