
Kristina Haase
Group Leader
ORCID: 0000-0003-3321-4286
EditEngineering vascularised tissue-specific disease models

Group Leader
ORCID: 0000-0003-3321-4286
EditMicrovessels are our earliest functional organs, and their dysfunction is linked to numerous diseases. The Haase group focuses on the development of novel in vitro human assays for examining the pathology of vascular disease, with a particular focus on those related to endocrine disorders and genetic differences stemming from sex. Our group has expertise in generating perfusable 3D in vitro vascular models. By incorporating self-assembled microvessels with tissue-specific cells, our capillary systems are used to investigate vasculogenesis (vessel formation) as well as microvascular pathologies. Our interdisciplinary group employs engineered platforms to investigate the role of physiological and pathological levels of mechanical cues on vascular development.
As tissue engineers, we employ a variety of fabrication, microscopy and biological techniques to ultimately increase our understanding of complex vascularized tissues. Our group works closely with clinicians and experts in modelling to develop physiological platforms for practical and predictive uses.
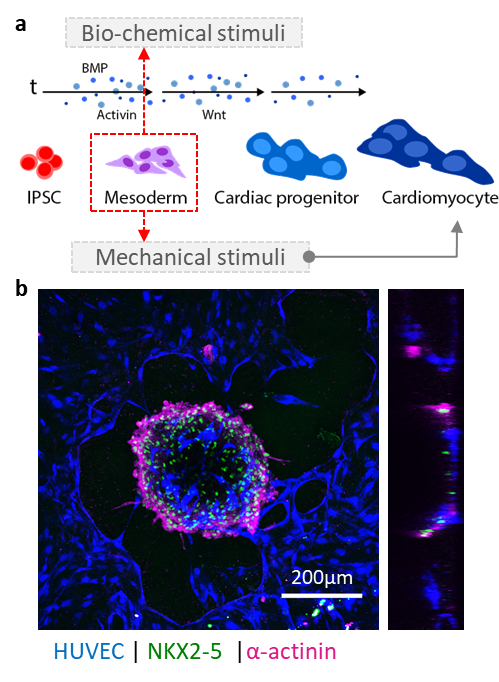
The Haase group develops strategies to vascularize organoids, spheroids and tissue grafts, with a particular focus on cardiac tissues. A long-term goal of the lab will be to implement our vascularized platforms for preclinical use.
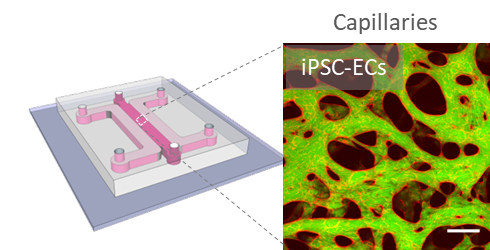
Tissue-specific microvascular models. We use our in vitro vessels to understand fundamental questions related to vascular development, remodeling and response to perturbations such as inflammation. Using human primary cells, we have generated models of placenta, cardiac, lung, and breast-specific microvessels on-chip. We are currently answering questions related to sex and hormonal influence.
Scalable human vasculature using induced pluripotent stem cells (iPSCs). By differentiating human iPSCs into endothelial cells and supporting stromal cell populations, our aim is to generate patient- and disease-specific vascular models. We are focusing on cardiac vascular models currently.
Vascularized tumors as physiological drug screening platforms. Integrating vasculature with tumor spheroids and patient-derived organoids facilitates investigation of heterotypic cell interactions, physiologically relevant drug dissemination, and prolonged culture of microtissues. We are characterizing the impact of tumors on microvessels and their surrounding matrix, using advanced imaging, nanoindentation and mass spectrometry. Our long-term goal will be to use these systems for interrogation using novel therapeutics.
Exploiting mechanical cues to promote vascular development. We are scaling down our microfluidic model and integrating controlled flow on-chip. We aim to achieve individually perfusable vascular units for answering questions related to flow-mediated vascular remodeling in tissue-specific vessels.

Insights from theoretical physics are helping scientists understand how living cells process information and use it to self-organise.
Edit
Lithuanian scientist began his three-year term chairing EMBL’s Council.
Edit
In the largest study of its kind, EMBL scientists reveal that certain microbes can thrive across different ecosystems, contributing to the global spread of antimicrobial resistance.
Edit
The Nordic EMBL Partnership for Molecular Medicine has appointed Janna Saarela as its new Speaker. Saarela is the Director of the Norwegian Centre for Molecular Biosciences and Medicine (NCMBM) and su…
Edit