Previous and current research
Chromatin, the faithful association of DNA with histone proteins, exists as the physiological form of our genome and the substrate for processes that regulate cellular gene expression. Numerous diseases, including neurodevelopmental disorders, are associated with mutations in genes that encode for chromatin-binding proteins or chromatin-modifying enzymes, which together act as epigenetic regulators. A central question grounding our research is how the normal/mutated epigenetic regulators engage in brain development, function and disease.
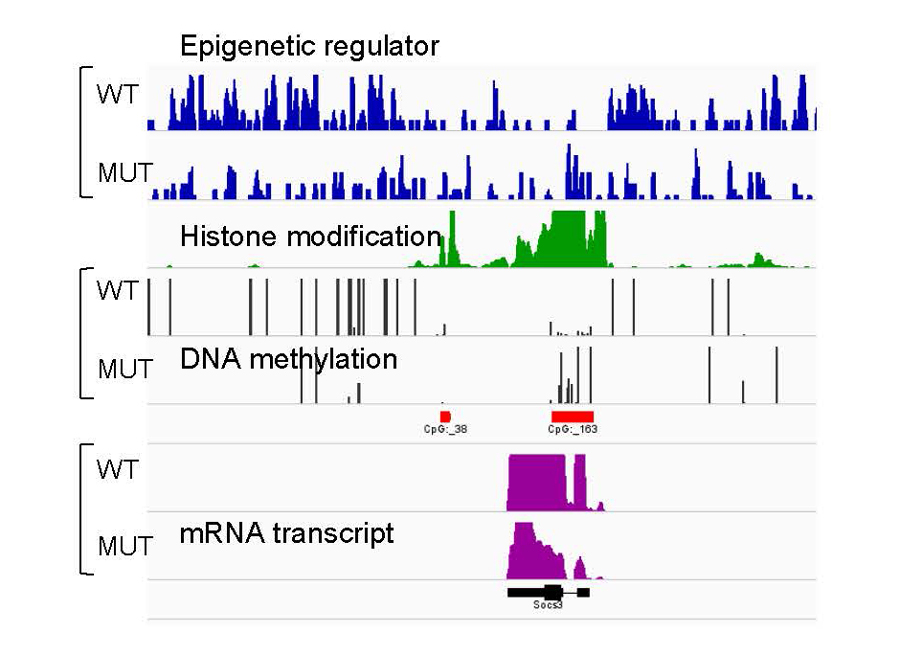
Histone variants and covalent modifications of both DNA and histones act in concert to define the landscape of our epigenome. Previously, we explored the interconnections between histone and DNA modifications by focusing on a conserved chromatin-binding regulatory domain, the ATRX-DNMT3-DNMT3L (ADD) domain. We showed that the ADD domain is capable of sensing, and therefore integrating, the status of multiple histone modifications. This in turn dictates the in vivo localisation of the full-length ADD-containing protein and its ability to function in downstream chromatin remodelling events. In parallel, we uncovered the localisation and function of a histone H3 variant, H3.3. Guided by distinct chaperone systems, H3.3 marks the genomic regions of histone turnover. We mapped the genome-wide localisation of H3.3 in mouse embryonic stem cells (mESCs) and neuronal precursor cells, and further expanded to terminally differentiated neurons to study its functional role in promoting neuronal plasticity.
Future projects and goals
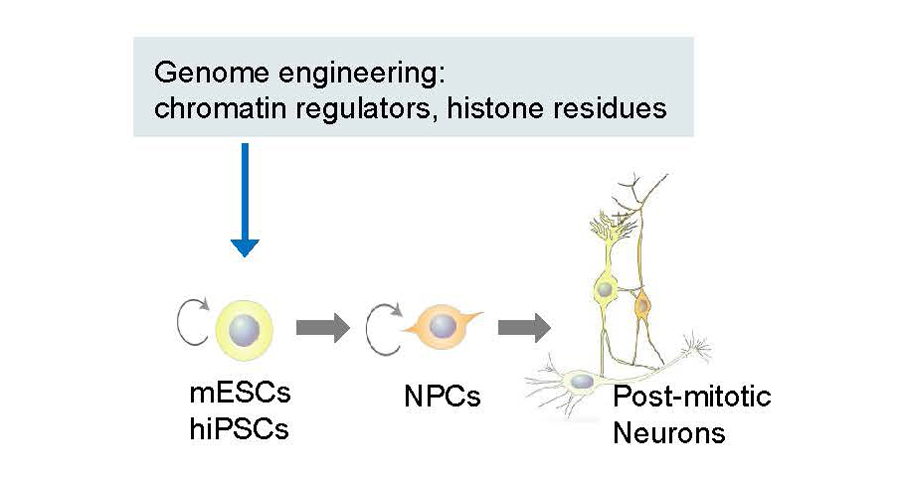
We aim to study chromatin regulation, its interpretation during brain development, and its misinterpretation in relation to brain cognitive and developmental disorders. We will focus on the molecular mechanisms that link genetic mutations encoded in epigenetic regulators to the widespread chromatin alterations. To model developmental stages and facilitate the necessary genome editing/engineering, we will use differentiating neurons from mESCs and human induced pluripotent stem cells (hiPSCs). Defining the ‘epigenetic landscape’ – both in normal and abnormal brain cells – will help provide novel targets for therapeutic intervention for cognitive and neurodevelopmental disorders.
Our research goals are to:
- Identify the location/function of mutated epigenetic regulators specific to cognitive deficits, and explore alterations of the epigenetic landscape in developing neurons.
- Investigate the functional roles of covalent modifications of DNA and histones in neurodevelopmental transcription programmes.