The SPC facility offers high quality services to external users at low price in order to cover the cost of consumables and maintenance of instruments.
Please contact SPC staff for pricing.
1) Mass Spectroscopy Services
Mass Spectrometry is a tool that has been widely used for investigations of topics in the realm of biophysics. We are equipped with a MALDI-TOF which is an valuable instrument for analytical characterization of proteins and peptides by measuring the mass-to-charge ratio of charged particles.
Services available:
- Protein assessment and MW determination
- Selenomethionine incorporation verification
- Crystal content verification
- Peptide mass fingerprinting
2) Thermal Denaturation Assay Services
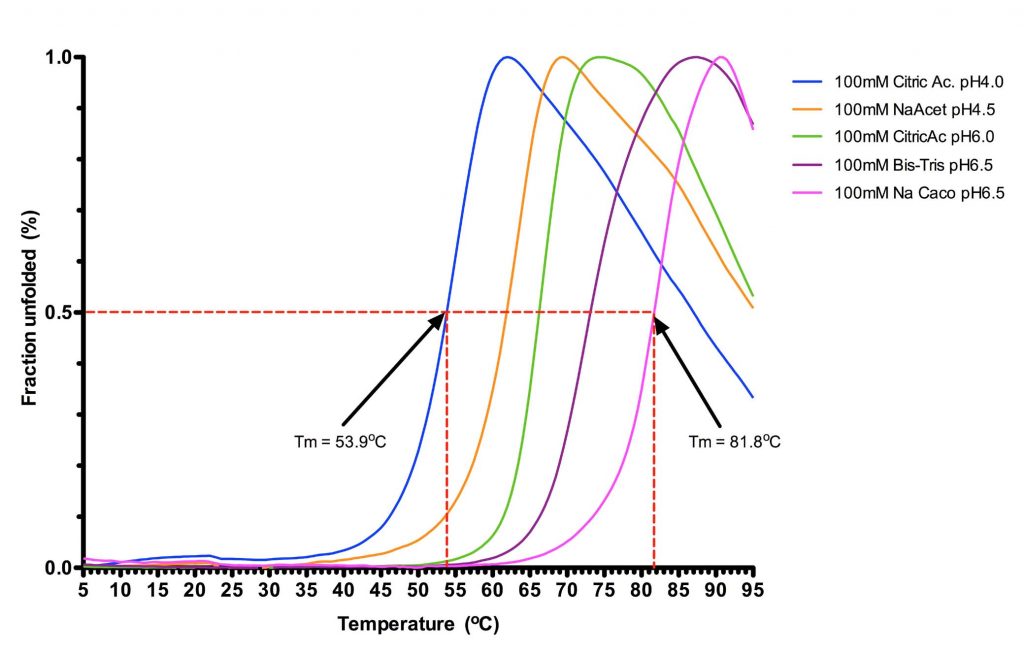
Target proteins used for crystallization can be studied by thermofluorescence shift assay (Thermofluor). In this method, the protein is incubated with a dye that only binds hydrophobic protein regions, where it becomes highly fluorescent. The protein is gradually heated, in order to slowly unfold, exposing these hydrophobic patches. The fluorescence signal is then used to determine the protein melting point (Tm). The value of Tm is influenced not only by the nature of the protein itself but also by its chemical environment (e.g., pH, salt nature and concentration, additives, presence of natural or synthetic ligands, etc.). It is possible determine optimum conditions stabilising the protein fold. It is known empirically that the more stable is a given protein (higher Tm) the more likely it is to crystallise.
The SPC Facility provides few screen plates which have been prepared in-house and set up using the HTX robot to assure precise pipetting and reproducibility. These screens are available and ready to use upon request.
SPC Screen 1: Sample stabilization screen 1 (buffers screen)
The screen 1 allow you to identify rapidly the best stabilizing condition under 96 different environments to optimise your purification buffer, binding buffer and storage buffer. Moreover, results may guide you in the decision to perform your purification or experiments, as well as your crystallization trial, at 4°C or 19°C.
More details about the SPC Screen 1
SPC Screen 2: Sample optimisation screen 2 (additives screen)
The screen 2 aims to explore the effect of several additives on the thermal stability of the proteins. We recommend to perform this screen to complement the screen 1 in order to identify an unknown ligand partner or increase the protein stability for the crystallisation trial.
More details about the SPC Screen 2
3) Dynamic Light Scattering (DLS) measurements
Dynamic light scattering (DLS) is a well-established technique for measuring the hydrodynamic radius (RH) and size distribution profile of molecules in solution. In particular it measures time-dependent fluctuations in the scattering intensity coming from particles undergoing random Brownian motion. Diffusion coefficient and particle size information can be obtained from the analysis of these fluctuations.
Sample requirements: 4-7ul of the sample at >0.25mg/ml
Please contact SPC staff for pricing.