Previous and current research
How do networks of genes control populations of cells to build organised tissues and organs? Organ development is a multi-scale process, in which molecular and cellular events control large-scale tissue movements, but these ‘macroscopic’ dynamics are equally important in feeding-back to regulate molecular events. A full understanding of how genes control organogenesis will thus require multi-scale computer modelling, and we have chosen vertebrate limb development as a model system to explore this problem.
Such a model should be based on quantitative data about dynamic tissue shapes and spatial distributions of gene activities. Traditional high-throughput and ‘omics’ technologies do not preserve spatial information, and we have therefore been developing and pioneering various 3D mesoscopic imaging technologies to generate geometric and spatial data for the model.
Our research thus falls primarily into two main areas:
- To further our understanding of organogenesis as a complex system, by bringing together a diverse range of techniques from biology, physics, imaging and computer science. Within this general theme, we focus on two aspects:
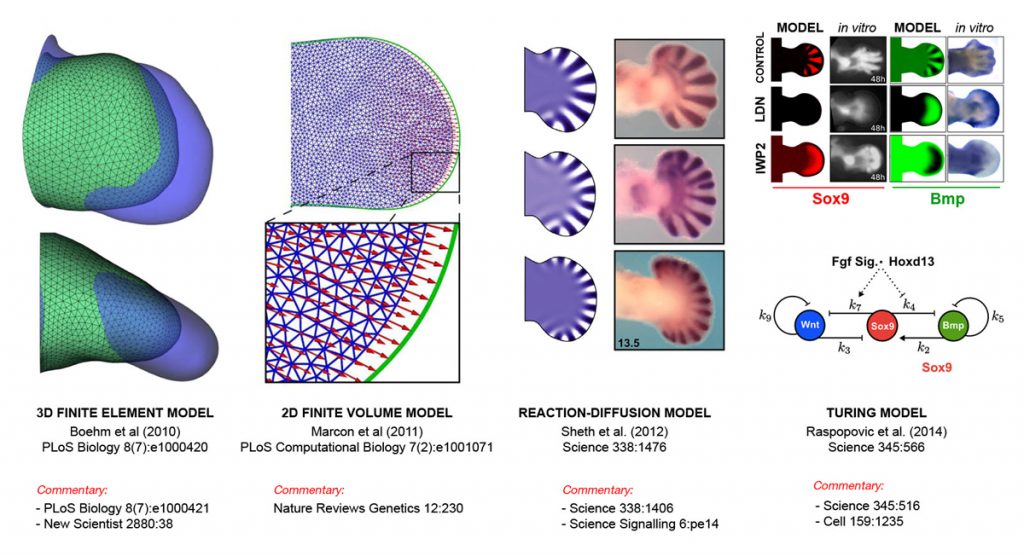
- We study a well-characterised standard model of development – the vertebrate limb/fin (using the mouse, chick and catshark). We combine experimental data (especially 3D data sets using optical projection tomography) within computational frameworks, to explore and test mechanistic hypotheses about how limb morphogenesis works. Using this approach we are studying both the physical morphogenesis (Boehm et al. 2010, PLoS Biol. and Marcon et al. 2011, PLoS Comp. Biol.) and also the genetic patterning mechanisms (Sheth et al. 2012, Science and Uzkudun et al. 2015 Molecular Systems Biology). We have used this mix of experimental and theoretical work to find evidence supporting the idea that digit patterning is achieved by a Turing reaction-diffusion system (Raspopovic et al. 2014, Science), and more recently have shown that this particular molecular systems has been conserved all the way from fish to mammals (Onimaru et al. 2016, Nature Communications).
- In addition to this specific model system, we are also interested in the theoretical principles by which gene regulatory networks can create controlled spatial patterns in multicellular contexts, both in a purely theoretical context (Cotterell et al. 2010, Molecular Systems Biology, Jimenez et al. 2015, PNAS, Jimenez et al. 2017, Molecular Systems Biology), and also in its application to synthetic biology (Schaerli et al. 2014, Nature Communications), and somite patterning (Cotterell et al. 2015, Cell Systems).
- Building on the success of the 3D imaging technique developed within the lab called Optical Projection Tomography (OPT – Science 296:541, 2002), the other major goal of the lab is to continue developing and improving 3D and 4D imaging technology including the development of time-lapse imaging of mouse limb development in-vitro (e.g. Nature Methods 5:609-12, 2008). Our recent projects include the development of OPTiSPIM (Maeyer et al., 2014).
Future projects and goals
- To create a full quantitative 4D ‘atlas’ of the distributed cellular activities (proliferation, integration, etc.) which together drive the mechanical process of limb bud morphogenesis.
- To develop realistic dynamic simulations of the gene regulatory circuits which drive the cellular activities and correct differentiation patterns.
- The long-term goal of the lab is to integrate the two phenomena described above into a full multi-scale model of mammalian limb development, successful enough to predict mutant phenotypes.
ERC ADVANCED INVESTIGATOR