Batch SAXS (static)
automated auto sampler
Automated BioSAXS at P12
allows users to perform experiments at a high turnover rate, including data collection and downstream processing.
Experiments are possible on-site or by mail-in access, where the beamline staff conducts them.
Three modules can be operated simultaneously:
Samples are measured in the vacuum Sample Exposure Unit, which houses a quartz capillary with a 1 or 2 mm diameter
automated auto sampler
Specifications:
size exclusion coupled SAXS
Specifications:
AF4 prior to SAXS
Specifications:
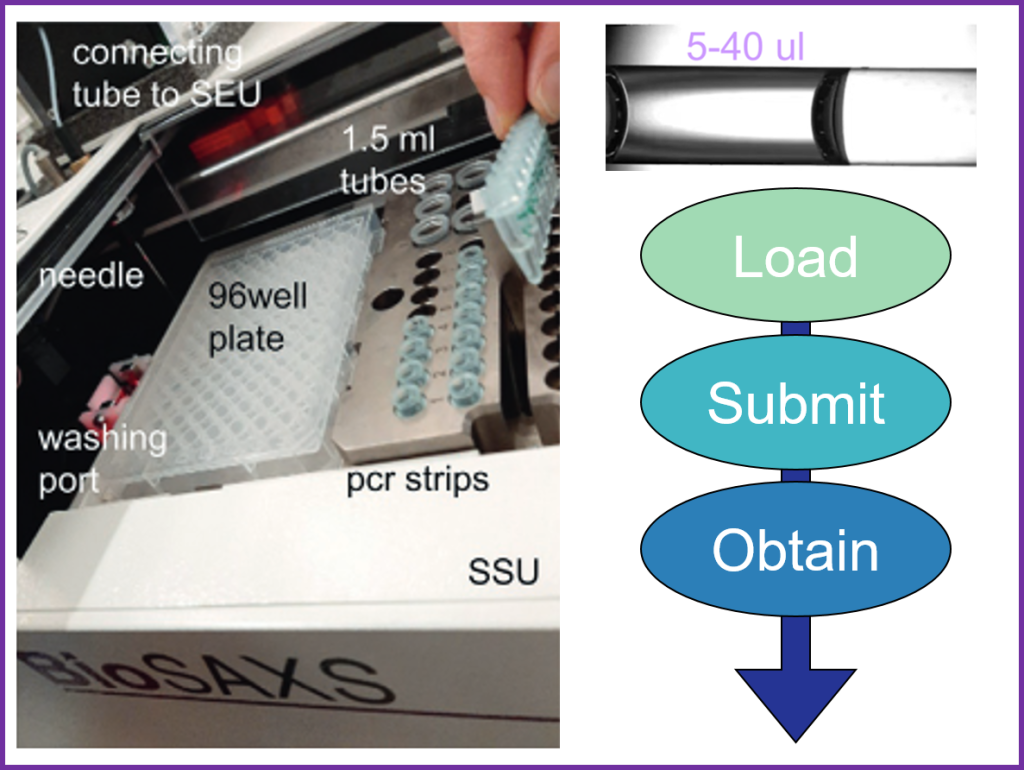
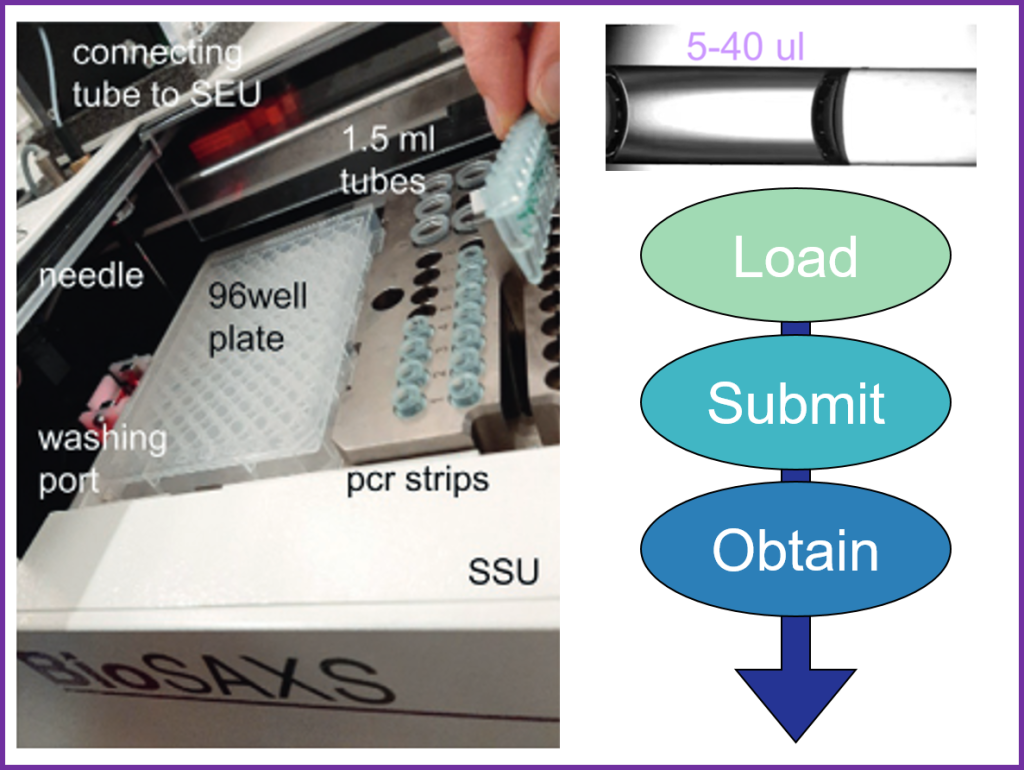
Batch measurements are conducted using the sample changer, a liquid-handling robot designed for SAXS data collection on protein solutions at equilibrium (EMBL-ARINAX).
The device can load as little as 5 µL of solution into a vacuum flow-through capillary, exposing the sample to the X-ray beam.
The sample continuously flows through the capillary to minimize radiation damage during exposure (20 ul loading volume required).
Between measurements, the system automatically cleans and dries the sample cell. A full cycle—including sample loading, exposure, cell cleaning, and drying—takes approximately 90 seconds.
The temperature of the sample cell and tray, where samples are stored before measurement, can be individually controlled.
The sample tray holds blocks for PCR strips (8 x 200 µl), 1.5ml tubes and 96-well plates. These consumables are available at the beamline.
A typical batch experiment involves measuring the sample at different concentrations, along with the corresponding buffer. Buffers are typically measured between each sample concentration to ensure accuracy.
Exposure times range from 1 to 5 seconds, depending on the sample’s radiation sensitivity. During this time, 20 to 50 frames are collected to identify any radiation damage so that compromised data can be discarded as needed.
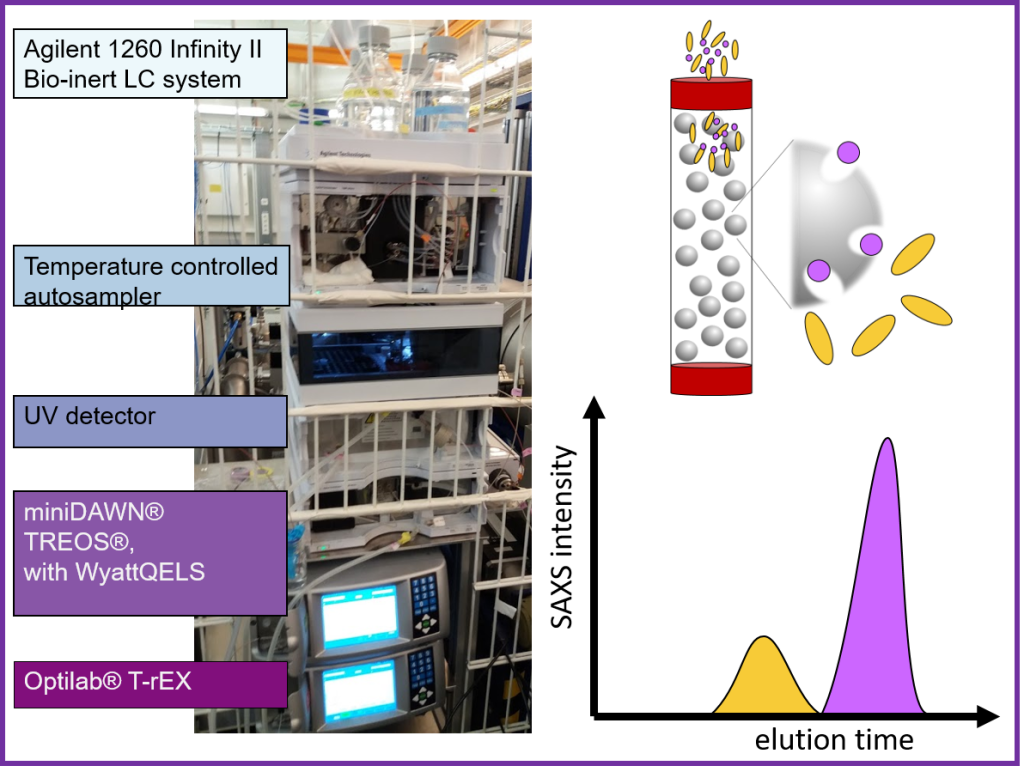
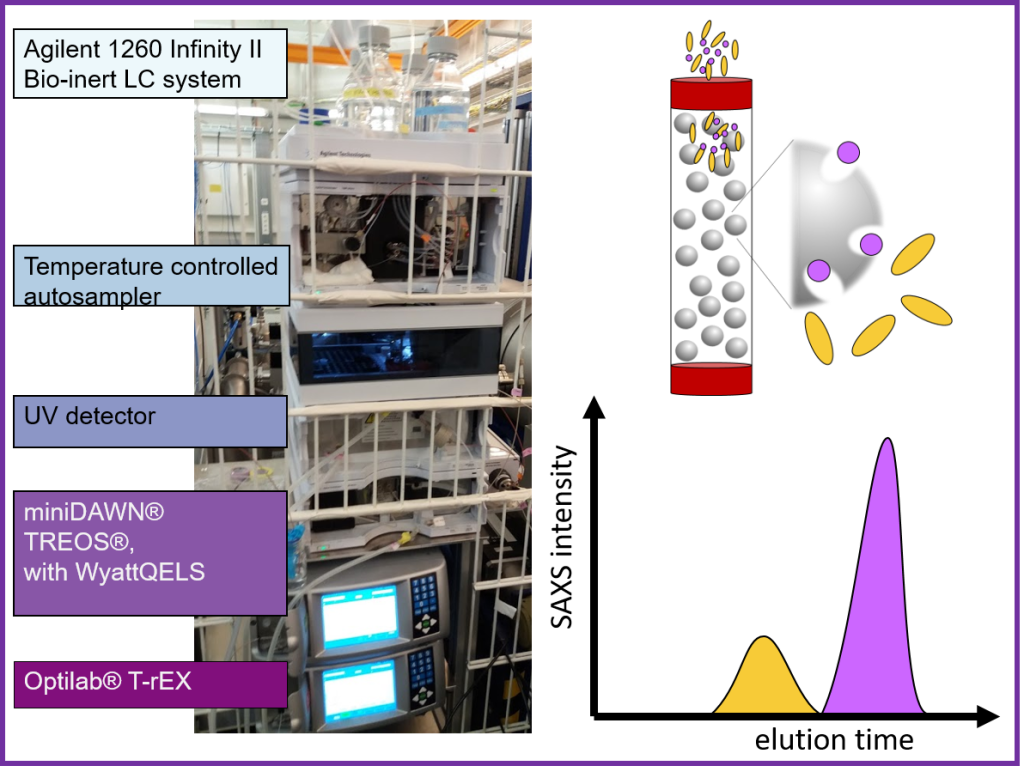
Size exclusion chromatography, in line with SAXS, SEC-SAXS, features an Agilent 1260 Infinity II Bio-Inert HPLC system with a temperature-controlled autosampler and a UV detector is a variable wavelength detector (VWD) ranging from 190-600 nm.
We provide Cytiva Superdex™ or Superose™ increase prepacked SEC columns. However, Users are encouraged to bring their columns, especially with specialized solvents, samples, or other columns containing different chromatography resins.
Duration of SEC-SAXS experiment is dependent on column separation. It ranges from 15 min (for 3ml column) to 50 min (24 ml column). During the whole elution process, SAXS frames are collected, where the exposure time ranges from 0.5 to 1 second.
The recommended injection can be between 20-100 µl depending on the sample concentration and selected column. As the sample is diluted during the separation process, higher sample concentrations are required, around 5-10 mg/ml. All measurements are possible at room temperature only.
In addition, we offer additional molecular weight validation from multi-angle laser light scattering (MALS) coupled to hydrodynamic radius measurements from dynamic light scattering (DLS) with the Wyatt miniDAWN TREOS/QELS instrument and a Optilab™ T-rEX refractive index (RI) instrument. When using the 24ml, this is done by splitting the eluent equally between detector array and SAXS capillary.
More information here:
Adding size exclusion chromatography (SEC) and light scattering (LS) devices to obtain high-quality small angle X-ray scattering (SAXS) data.
Graewert MA, Da Vela S, Gräwert TW, Molodenskiy DS, Blanchet CE, Svergun DI, Jeffries CM
Crystals, 2020
doi:10.3390/cryst10110975.
List of available columns provided:
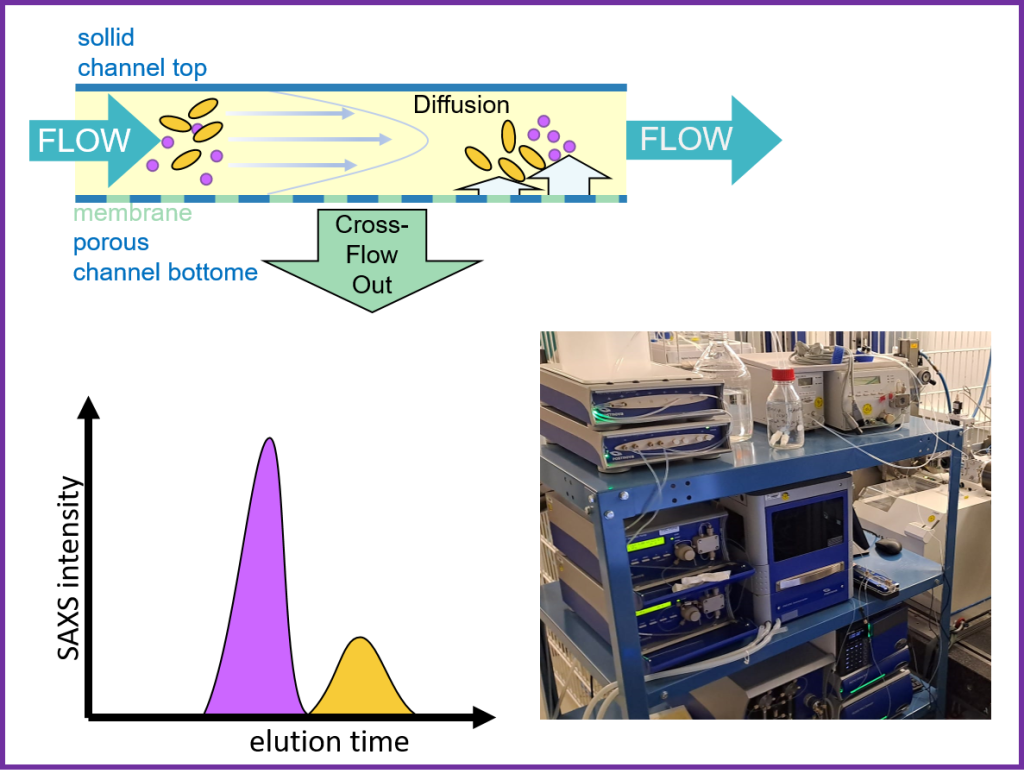
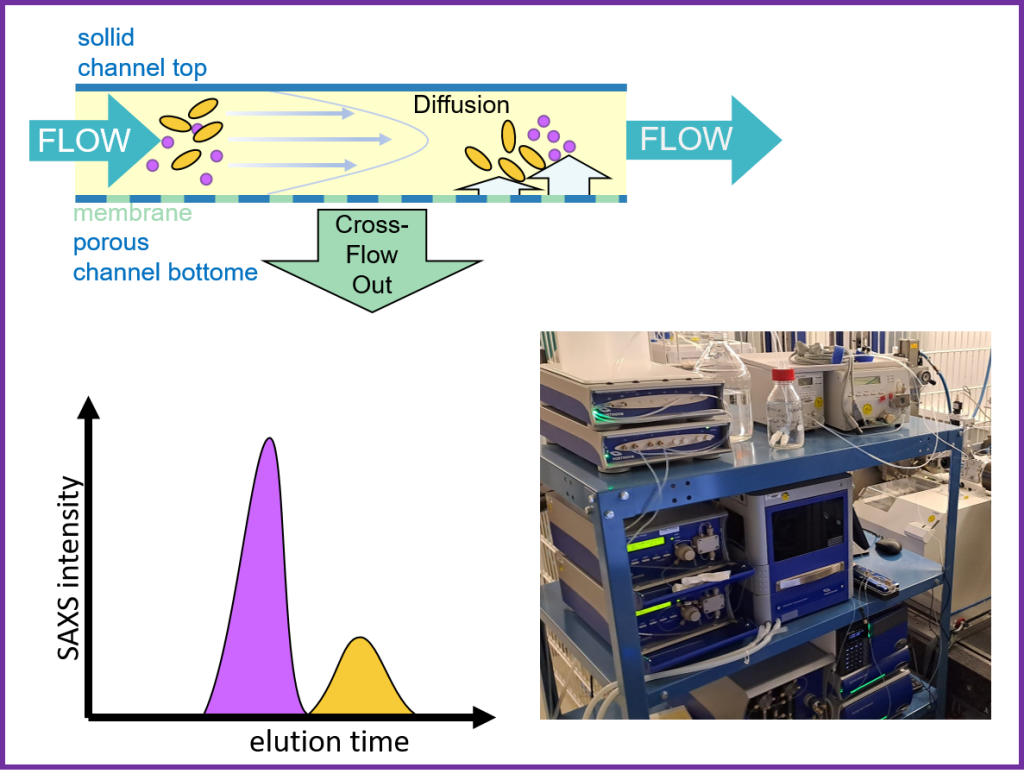
Asymmetric Flow Field-Flow Fractionation, in line with SAXS, AF4-SAXS features a Postnova AF2000 MultiFlow FFF coupled with the P12 beamline for in situ SAXS measurements. This setup allows for precise fractionation of macromolecules, aggregates, and nanoparticles in the aqueous phase.
A wide variety of sample types, including proteins, polymers, nanoparticles, and large macromolecular complexes, can be processed, offering unique advantages in separating macromolecules from aggregates or other components that may interfere with SAXS signal analysis.
The sample is injected into the AF4 system using the autosampler, separating the components. After fractionation, The mobile phase goes through the corresponding detectors Postnova Detectors PN3211 UV-Vis for absorbance measurements, PN3609 Multi-Angle Light Scattering (MALS) for molecular weight determination, and PN3712 Dynamic Light Scattering (DLS) for hydrodynamic radius measurements, through the elution peaks, providing a comprehensive analysis of the sample.
(Currently a additional set-up with Wyatt components is under commission and accessible upon request)
Before obtaining beamtime, the AF4 separation method must be established. This must be prearranged with bemaline staff.
AF4-SAXS experiments typically require 30-60 min per run, with a recommended exposure time of 0.5 to 2 seconds.
Set-up described in detail here:
Quantitative size-resolved characterization of mRNA nanoparticles by in-line coupling of asymmetrical-flow field-flow fractionation with small angle X-ray scattering.
Graewert MA, Wilhelmy C, Bacic T, Schumacher J, Blanchet C, Meier F, Drexel R, Welz R, Kolb B, Bartels K, Nawroth T, Klein T, Svergun D, Langguth P, Haas H
Scientific reports, 2023
doi:10.1038/s41598-023-42274-z.