Collective action
Occupied genetic switches hold clues to cells’ history
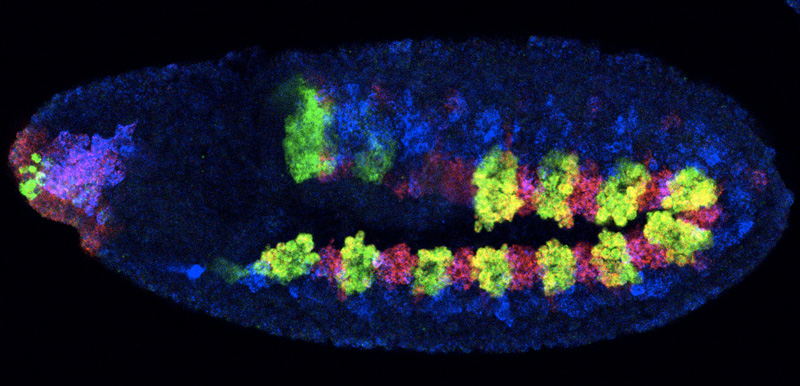
If you wanted to draw your family tree, you could start by searching for people who share your surname. Cells, of course, don’t have surnames, but scientists at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, have found that genetic switches called enhancers, and the molecules that activate those switches – transcription factors – can be used in a similar way, as clues to a cell’s developmental history. The study, published today in Cell, also unveils a new model for how enhancers function.
Looking at fruit fly embryos, Guillaume Junion and Mikhail Spivakov, collaborating scientists in the groups of Eileen Furlong at EMBL and Ewan Birney at EMBL’s European Bioinformatics Institute (EMBL-EBI), found that, in heart muscle cells, enhancers which are meant to be active aren’t the only ones that have groups of transcription factors attached. Surprisingly, enhancers that should be active only in the neighbouring gut muscle were also occupied by transcription factors in heart cells.
“Although it may seem counter-intuitive to leave unnecessary genetic switches available for activation and then have to actively suppress them, the findings make sense in developmental terms,” says Furlong.
Both heart and gut muscle cells develop from the same pool of precursor cells. Enhancers for both groups seem to be made available to transcription factors in the precursor cells, before they ‘grow up’ to be either heart or muscle cells. If this is the case, scientists could work out the relationships between cells by looking at what occupied enhancers they share.
Intriguingly, heart muscle cells don’t actually have the transcription factors that bind to gut enhancers in gut muscle cells. Instead, the gut enhancers in heart cells were occupied by transcription factors produced only by the heart.
Furlong and colleagues found that transcription factors are able to attach themselves to enhancers in groups, with some transcription factors binding directly to the enhancer’s DNA and others binding to those enhancer-bound transcription factors. This means that the genetic sequence of these enhancers can vary greatly, yet they are occupied as a united group – a strategy that differs from the two ways in which enhancers were already known to function. This flexibility in the enhancer’s genetic sequence means that it can mutate without disastrous effects, giving it some evolutionary flexibility.
The EMBL scientists are now investigating how far that flexibility extends. They are looking at variation between species, extending their studies to another species of fruit fly, Drosophila virilis, which is, genetically speaking, as different from the commonly-used Drosophila melanogaster as humans are from chickens.
Source article
Junion, G., Spivakov, M., Girardot, C., Braun, M., Gustafson, E.H., Birney, E. & Furlong, E.E.M. A transcription factor collective defines cardiac cell fate and reflects the developmental history of this cell lineage. Cell, 3 February 2012.
DOI:10.1016/j.cell.2012.01.030.



