Locating ground zero
How the brain’s emergency workers find the disaster area
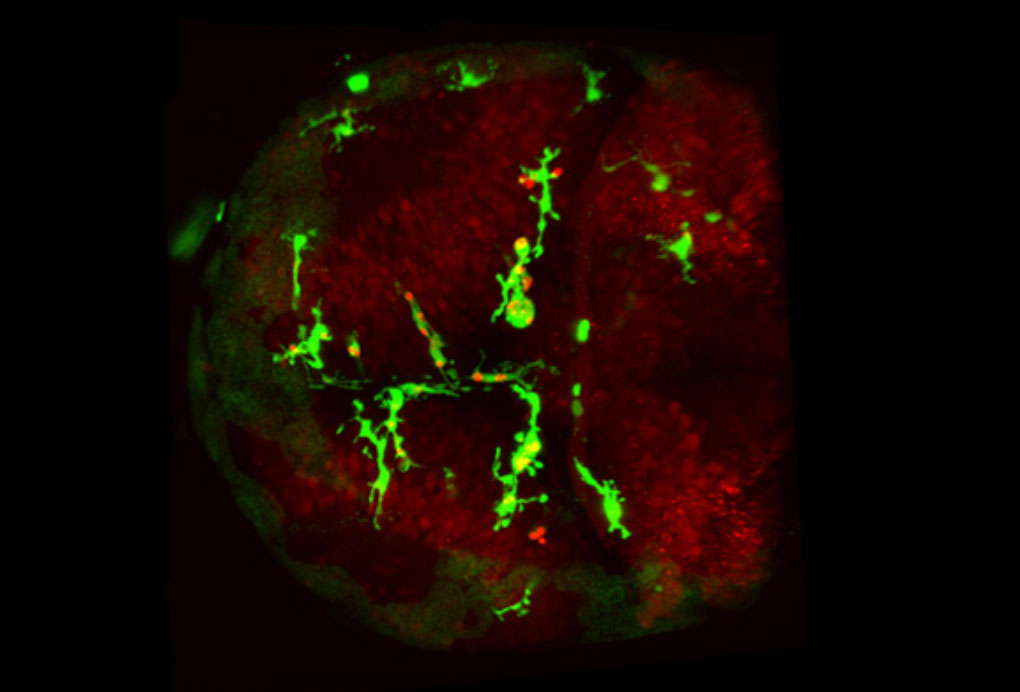
Like emergency workers rushing to a disaster scene, cells called microglia speed to places where the brain has been injured, to contain the damage by ‘eating up’ any cellular debris and dead or dying neurons. Scientists at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, have now discovered exactly how microglia detect the site of injury, thanks to a relay of molecular signals. Their work, published today in Developmental Cell, paves the way for new medical approaches to conditions where microglia’s ability to locate hazardous cells and material within the brain is compromised.
“Considering that they help keep our brain healthy, we know surprisingly little about microglia,” says Francesca Peri, who led the work. “Now, for the first time, we’ve identified the mechanism that allows microglia to detect brain injury, and how that emergency call is transmitted from neuron to neuron.”
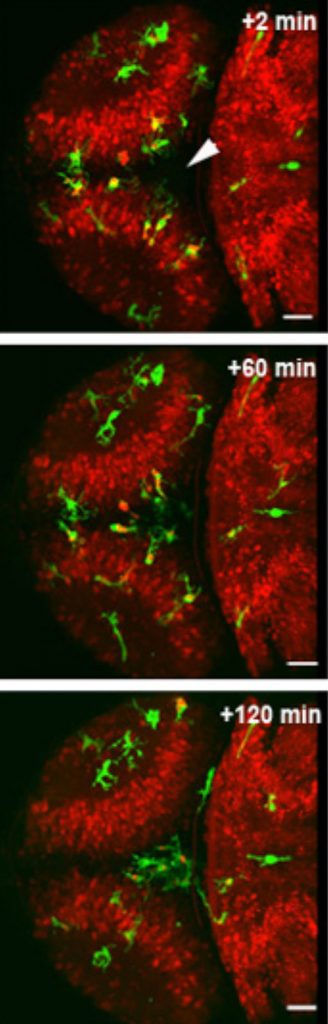
site of injury (arrow) to clear up debris.
Video
When an emergency occurs, cries can alert bystanders, who will dial the emergency number. A call will go out over the radio, and ambulances, police or fire engines in the area will respond as needed. In the brain, Peri and colleagues found, injured neurons send out their own distress cry: they release a molecule called glutamate. Neighbouring neurons sense that glutamate and respond by taking up calcium. As glutamate spreads out from the injury site, this creates a wave of calcium swallowing. Along that wave, as neurons take up calcium they release a third molecule, called ATP. When the wave comes within reach, a microglia cell detects that ATP and takes it as a call to action, moving in that direction – essentially tracing the wave backwards until it reaches the injury.
Scientists knew already that microglia can detect ATP, but this molecule doesn’t last long outside of cells, so there were doubts about how ATP alone could be a signal that carried far enough to reach microglia located far from the site of injury. The trick, as Peri and colleagues discovered, is the long-lasting glutamate-driven calcium wave that can travel the length of the brain. Thanks to this wave, the ATP signal is not just emitted by the injured cells, but is repeatedly sent out by the neurons along the way, until it reaches microglia.
Dirk Sieger and Christian Moritz in Peri’s lab took advantage of the fact that zebrafish have transparent heads, which allow scientists to peer down a microscope straight into the fish’s brain. They used a laser to injure a few of the fish’s brain cells, and watched fluorescently-labelled microglia move in on the injury. When they genetically engineered zebrafish to make neurons’ calcium levels traceable under the microscope, too, the scientists were able to confirm that when the calcium wave reached microglia, these cells immediately started moving toward the injury.
Knowing all the steps in this process, and how they feed into each other, could help to design treatments to improve microglia’s detection ability, which go awry in conditions such as Alzheimer’s and Parkinson’s diseases.
Watch Francesca Peri, Dirk Sieger and Christian Moritz discuss their work and its implications in this short film produced as a Cell video abstract: youtu.be/fbdT_-yhakQ.
Source article
Sieger, D, Moritz, C., Ziegenhals, T., Prykhozhij, S. & Peri, F. Long-range Ca2+ waves transmit brain damage signals to microglia. Developmental Cell, published online 24 May 2012.
Article abstract
Microglia are the resident phagocytes of the brain that are responsible for the clearance of injured neurons, an essential step in subsequent tissue regeneration. How death signals are controlled both in space and time to attract these cells towards the site of injury is a topic of great interest. To this aim, we have used the optically transparent zebrafish larval brain and identified rapidly propagating Ca2+ waves that determine the range of microglial responses to neuronal cell death. We show that while Ca2+-mediated microglial responses require ATP, the spreading of intercellular Ca2+ waves is ATP independent. Finally, we identify glutamate as a potent inducer of Ca2+-transmitted microglial attraction. Thus, this real-time analysis reveals the existence of a mechanism controlling microglial targeted migration to neuronal injuries that is initiated by glutamate and travels across the brain in the form of a Ca2+ wave.
Press Coverage
- THE SCIENTIST , 1 SEPTEMBER 2012Finding Injury



