SPIM doctors
Any high school student has probably watched little bugs bumping around under a microscope. Looking inside those organisms is trickier, but EMBL scientists continue to push the boundaries of what microscopes can do.
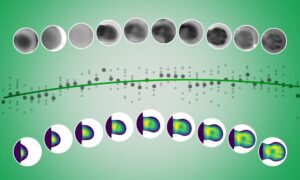
The invention of SPIM, which allowed scientists to watch an embryo develop for the first time, was hailed as one of the top breakthroughs. Selective Plane Illumination Microscopy (SPIM) is still relatively new, only dating back to the mid 1990s. But its huge potential is already clear. What started in a physics lab led to the first groundbreaking insights in biology just 10 years later. Now, with new papers and a new start-up, EMBL researchers show they are still adding new dimensions to the technology.
Let there be light
It was in the late 1980s that Roelof W Wijnaendts-van-Resandt, Ernst Stelzer and members of his lab at EMBL started developing the multi-lens microscopes that would become SPIM.
The simplest microscopes shine light on or through a subject, which an observer then looks at through a magnifying lens. Fluorescence microscopy — itself a relatively new technique — uses dyes or genetic modifications that cause specific parts of a cell to glow when exposed to certain wavelengths of light.
Fluorescence microscopy is powerful, but the light that spurs cells to fluoresce also damages them. Stelzer’s lab found a solution that has unlocked huge opportunities. By confining light to a very thin sheet, they could light up just one plane at a time for the microscope to focus on, protecting the other parts of the sample. Repeating the process over and over gives a complete picture of the specimen.
‘Intellectual breakthrough’
Jan Huisken was a PhD student in Stelzer’s lab in the early 2000s. A decade earlier, Stelzer’s lab had developed a fluorescence microscope that used two opposing lenses to get a full view of a sample suspended in a droplet of water. Huisken’s task was to use beams of light to manipulate a sample suspended in a water droplet — something that turned out to be very difficult. The group decided to look for an easier solution, and SPIM was born.
“There are moments when you can really say, ‘That is an intellectual breakthrough’,” says Stelzer, who is now at the Buchmann Institute for Molecular Sciences at the Goethe Universität in Frankfurt. It wasn’t long before Stelzer and his biologist colleagues were seeing the possibilities of SPIM.
An advanced version of SPIM was used to create the stunning videos of a growing fish embryo highlighted by Science as one of the breakthroughs of the year in 2008. EMBL biophysicist Phillip Keller’s movie let scientists track each individual cell, answering previously unanswerable questions about how animals develop.
The ability to rapidly image a large three-dimensional sample and the small amount of light used —SPIM’s unique properties — make it possible to watch an entire living organism in exquisite detail.
“People are naturally limited by the tools that they have available,” says Keller, who is now at Howard Hughes Medical Institute’s Janelia Research Campus. With SPIM, he and other researchers can take entirely new approaches to research questions.
Software upgrade
Innovation didn’t stop there. SPIM is powerful, but the light can’t penetrate fully into larger samples, making for a blurry picture. Lars Hufnagel, a group leader at EMBL since 2007, helped develop a new technique called multi-view-SPIM, or MuVi-SPIM. It uses two light sheets on opposite sides of the sample and two imaging lenses to create a much clearer picture in much less time than on original SPIMs, where samples had to be rotated to look into them from multiple sides. Hufnagel’s lab has since further improved upon this principle
Hufnagel described the key to that advance with his colleagues in a Nature Communications paper last month. In previous versions of this technique, the light sources had to illuminate the sample one at a time or the detector would be overwhelmed by scattered light.
Now, Hufnagel, in collaboration with Hamamatsu Photonics, has written a new computer program for the cameras that makes it possible to have both lights shine at the same time and detect only unscattered light. In addition to improved image quality, this doubles the speed of the experiment and halves the number of images the computer has to store and analyze. “The nice thing about this is it is just a software upgrade on the camera,” he says.
Inverted SPIM
The MuVi-SPIM technology is being marketed by a new start-up called Luxendo. Hufnagel and Jan Ellenberg founded the company with EMBL’s technology transfer arm, EMBLEM.
“We were contacted by a number of labs that wanted to get access to this disruptive technology,” says Juergen Bauer, EMBLEM’s deputy managing director. That demand inspired him to work with the EMBL scientists to create Luxendo.
Now Ellenberg and Hufnagel are working to take SPIM even further. Their latest paper, just published in Nature Methods, makes use of a development called inverted SPIM. The standard SPIM setup has the detector and light parallel to the lab bench. That means the sample has to be held in place vertically in a capillary tube or a gel. Embedding the biological sample in that very unnatural environment means fragile samples like mammal embryos couldn’t be imaged.
In inverted SPIM, the lenses point up at an angle from underneath the specimen, which simply rests on a glass slide like a conventional microscope. Without the need to embed and suspend the sample, the system is much easier to use. It might sound like a simple improvement, but it could answer questions about infertility. Many embryos fail in their very early stages after only dividing a few times. Some eggs don’t even make it to fertilization before they die.
You have to be able to look inside the cells when they are having the problems.
“We don’t understand why that is, why the divisions aren’t happening properly. You have to be able to look inside the cells when they are having the problems,” Ellenberg says.
His paper describes the first two days of a mouse embryo’s development imaged with inverted SPIM. Mammal embryos die when embedded in gels or tubes for conventional SPIM, but inverted SPIM overcomes that problem, enabling scientists to watch live a process that they could thus far only speculate upon. The new research is a starting point for learning more about mouse embryos, which will be important to understand a major reason for human infertility.
Double trouble
Ellenberg and Hufnagel are planning to develop a multi-view version of this technique as well, but for now they say that simply doubling the number of lenses makes things a bit too crowded. Instead, they are working on more advanced optical designs.
Considering SPIM’s growth so far, it seems almost inevitable that somebody will soon solve that problem. It’s the kind of improvement that seems especially possible at a place like EMBL. Hufnagel says the twin missions of developing new technology and advancing the life sciences are what make EMBL suited to breakthroughs like SPIM. The many improvements and derivations EMBL researchers continue to make are further testament to that.