Plasticity of the binding pocket in peptide transporters underpins promiscuous substrate recognition.
Cell Reports 18 July 2023
10.1016/j.celrep.2023.112831
Issue 101
The Löw Group at EMBL Hamburg explores the structures of promiscuous molecules critical for nourishment and drug absorption
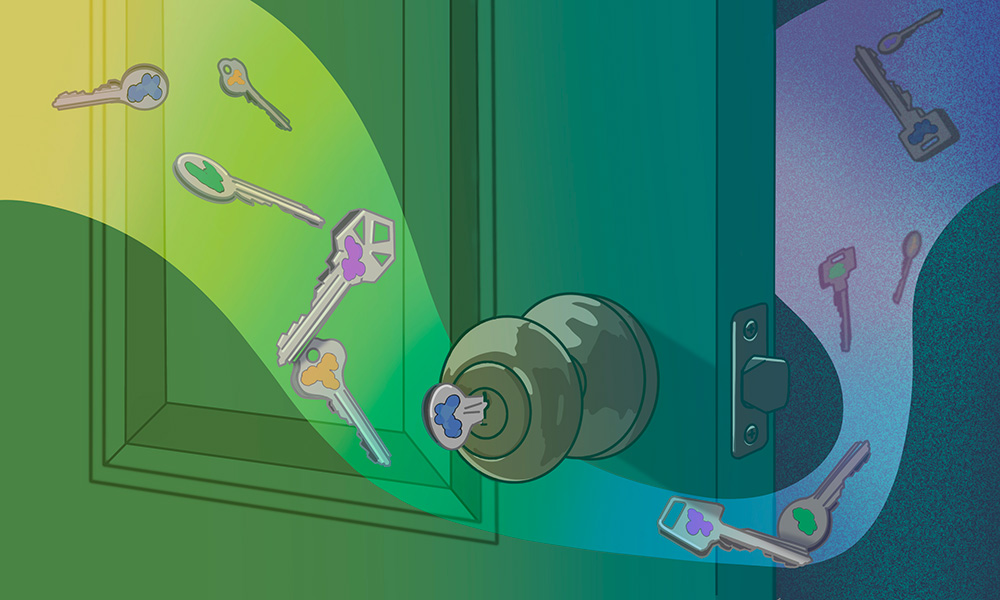
How do cells eat? This question lies at the focus of research undertaken by the Löw Group at EMBL Hamburg and Centre for Structural Systems Biology (CSSB). Using structural biology methods, they explore how ‘promiscuous’ proteins enable cells to absorb nutrients, and how this could be used to make drug uptake more efficient in the future.
To survive, living cells absorb nutrients from their environment. Their menu includes various delicacies, such as sugars, fats, and peptides, which are tiny pieces of digested proteins that cells use to build their own proteins. To capture and pull these nutrients inside, cells use dedicated transporter molecules that sit in the cell membrane.
Many molecular transporters are highly specialised, e.g. they only transport one type of sugar. But it’s different for peptides – and that’s where promiscuity comes into play.
Peptide transporters known as POTs (proton-coupled oligopeptide transporters) are not picky at all. In fact, they’ll grab almost any peptide they find in their way, regardless of its composition and shape. This ability is described by structural biologists as ‘promiscuity’.
“How promiscuity works has been one of the main questions in structural biology,” said Christian Löw, Group Leader at EMBL Hamburg and Centre for Structural Systems Biology (CSSB). “POTs are especially fascinating, because they are much more promiscuous than most other transporters. You could compare them to a lock that can be opened by many different keys. I wanted to learn how this works.”
The Löw Group are experts in structural biology of membrane proteins, in particular promiscuous nutrient transporters. Their work on different POTs, ranging from those in bacteria to those in humans, has yielded many insights that may help solve the mystery.
The Löw Group explored this further in their recent work in collaboration with the Marquez Team at EMBL Grenoble and the Steyaert Lab at the Vrije Universiteit Brussel. They determined and compared the X-ray structures of a bacterial POT called DtpB while it was bound to 14 dietary peptides of different sizes, shapes, and chemical properties. They were surprised to see that during the transport, DtpB undergoes major structural changes to adapt itself to each peptide, while the structure of the peptides themselves remains largely unchanged.
“You could compare it to a vehicle that wraps itself around the passenger to transport them. In the world of molecular biology, this is very counterintuitive,” said Löw.
Further experiments brought more surprises as they showed that the peptides that most strongly bind to DtpB are poorly transported. The peptides transported most efficiently were actually the ones with moderate binding strength.
“Strong binding is like superglue that gets the peptides stuck inside DtpB and block the passage for other peptides,” said Katharina Jungnickel, postdoc in the Löw Group.
This pattern most likely also applies to POTs in humans and other species, such as those that transport dietary peptides from the gut into the bloodstream – which the Löw Group studied as well. In fact, the scientists expect that ‘moderate binders are best’ could be a more general feature of promiscuous transporters across organisms.
Understanding POTs’ promiscuity may be key for improving drug design.
POTs transport many peptide-like drugs. For example, in the human gut, POTs are responsible for the uptake of various drug molecules, e.g. some drugs for hypertension, while in bacteria, they may serve as an entry point for certain antibiotics.
However, this transport is often inefficient, so high drug doses are needed. This in turn may cause more side effects. Many potentially effective drugs might not even get transported to the right places in the body. This is one among several reasons why drugs fail in clinical trials.
“If we could predict which drugs will be transported at early stages of drug development, this would save a lot of time and money,” said Vadim Kotov, former postdoc in the Löw Group, now working in industry. “That’s why we tried to crack the code that determines which peptides gets transported.”
To pursue this, the scientists combined experiments with machine learning. To their surprise, the analysis revealed that DtpB is much less promiscuous than thought before – out of the 8400 possible di- and tripeptides, it is likely to bind only a few hundred. They also identified a few factors, such as peptide size, charge and chemical properties, that are key for peptide recognition.
Using this information, the scientists have built a bioinformatics pipeline that could be used for other POTs to predict which peptides might potentially get transported and which definitely not. Eventually, this could help the pharmaceutical industry exclude poorly absorbed drugs at earlier stages of drug development. However, for more precise predictions, more research is necessary.
“It’s still a mystery what exactly the peptides need in order to be transported,” said Jungnickel. “Although it’s a relatively simple system, it’s harder to figure out than we thought.”
“Arguably, an even larger dataset would be necessary to train next-generation predictive models,” added Kotov.
Another recent study by the Löw Group in collaboration with colleagues from Boehringer Ingelheim dives into the function of another POT, called PHT1 (peptide/histidine transporter 1). It is found in the membrane of lysosomes, cell organelles involved in ‘digesting’ defective and worn-out cellular components, among other functions. PHT1 is quite peculiar because besides transporting peptides, it can also detect signals and trigger molecular reactions inside the cell. Proteins with this ability are called receptors.
“We never expected that PHT1 could function as a receptor,” said Tânia Custódio, postdoc in the Löw Group. “POTs are predicted to have similar transport mechanisms and having a dual transport-receptor function was unheard of. I was really curious to understand the function of this peptide transporter in our immune system.”
While PHT1 is important for our immunity, overactivity of its receptor function may lead to autoimmune diseases, such as systemic lupus erythematosus, as well as to inflammatory bowel diseases and type 2 diabetes. Blocking PHT1 receptor function might, therefore, help treat systemic lupus erythematosus.
Designing such blocker molecules requires knowing the detailed structure of PHT1. To enable this, the Löw Group determined the molecular structure of PHT1 and mapped its interaction surface with another protein that helps PHT1 to trigger the molecular response to signals.
This model can serve as a guide for other researchers to develop molecules that would either block peptide transport or the receptor function of PHT1.
“Ten years of POT research at EMBL – from bacteria to humans and back – during which we obtained fantastic molecular insights into the structure and mechanisms of this important transporter family,” said Löw. “The research opens up new avenues in academic settings and pharmaceutical industry. Our findings can be used to modify existing and future drugs for improved uptake or to develop small molecules to inhibit the PHT1’s receptor function. The future looks bright for transporter research.”
Cell Reports 18 July 2023
10.1016/j.celrep.2023.112831
Nature Communications 14 September 2023
10.1038/s41467-023-41420-5

Large language models are changing the way we carry out scientific data curation, annotation, and research, setting the stage for a more efficient understanding of scientific literature

With the new advanced mobile laboratory, EMBL is taking its service offerings to new heights, bringing cutting-edge life science technologies to the field in a way never seen before.

Click here to download a quick overview of all the articles included in this digital issue of EMBLetc.