Celebrating 100 issues of EMBLetc.
First published in 1999 as a black-and-white printed newsletter for EMBL staff and alumni, EMBLetc. has undergone many transformations in its 24 years of existence.
Issue 100
EMBL Barcelona researchers are studying how tissues develop both in healthy and diseased states using organoids and 3D multicellular systems to mimic human organs and their functions.
Our body is influenced greatly by the context within which it lives. The food we eat, the air we breathe, and the environment around us, all affect the way our body functions and responds to challenges. Researchers at EMBL Barcelona aim to understand the importance of such context in disease development. To achieve this, they are increasingly making use of groundbreaking technologies like organs-on-chip and organoids, which have the potential to revolutionise the way we study, diagnose, and treat diseases.
Organs-on-chip – also called microphysiological systems (MPS) – are small cell-based devices, which can range from the size of a one-cent coin to that of a credit card. They allow researchers to recreate the structures and functions of human organs on a miniature scale.
Organoids, on the other hand, are three-dimensional cell aggregates which can grow up to a couple of millimetres in size. They mimic aspects of the architecture or function of real organs, such as the heart or the brain.
Together, these technologies offer a powerful new way to study how diseases develop, test drugs, and potentially develop personalised therapeutic treatments.
Since organoids and MPS can mimic organ and tissue function in the laboratory, they are an invaluable tool for researchers trying to understand organ function and dysfunction under controlled conditions. At EMBL Barcelona, Maria Bernabeu, Talya Dayton, Miki Ebisuya, and Kristina Haase lead research groups that study cerebral malaria, cancer, spinal deformation, and microvascular dysfunction, respectively, using these systems.
Maria Bernabeu and her team study cerebral malaria, a disease that causes around 400,000 deaths a year. Malarial parasites stick to small blood vessels in the brain (microvasculature) releasing toxins that disrupt the blood-brain barrier, causing vessel blockage and brain swelling. This makes cerebral malaria one of the most fatal malaria complications with a 20% mortality rate even after antimalarial drug administration.
“Current knowledge of cerebral malaria is based primarily on autopsy analysis, because of limitations of available animal models,” said Viola Introini, postdoc in the Bernabeu Lab. “We cannot study disease onset and progression in humans because of ethical reasons. As an alternative, our group engineers 3D human brain microvessel models that incorporate crucial cells like pericytes and astrocytes to better mimic the blood-brain barrier. They can be used to study vascular dysfunctions caused by parasite infection in physiological conditions, aiming to provide a holistic understanding of cerebral malaria.”
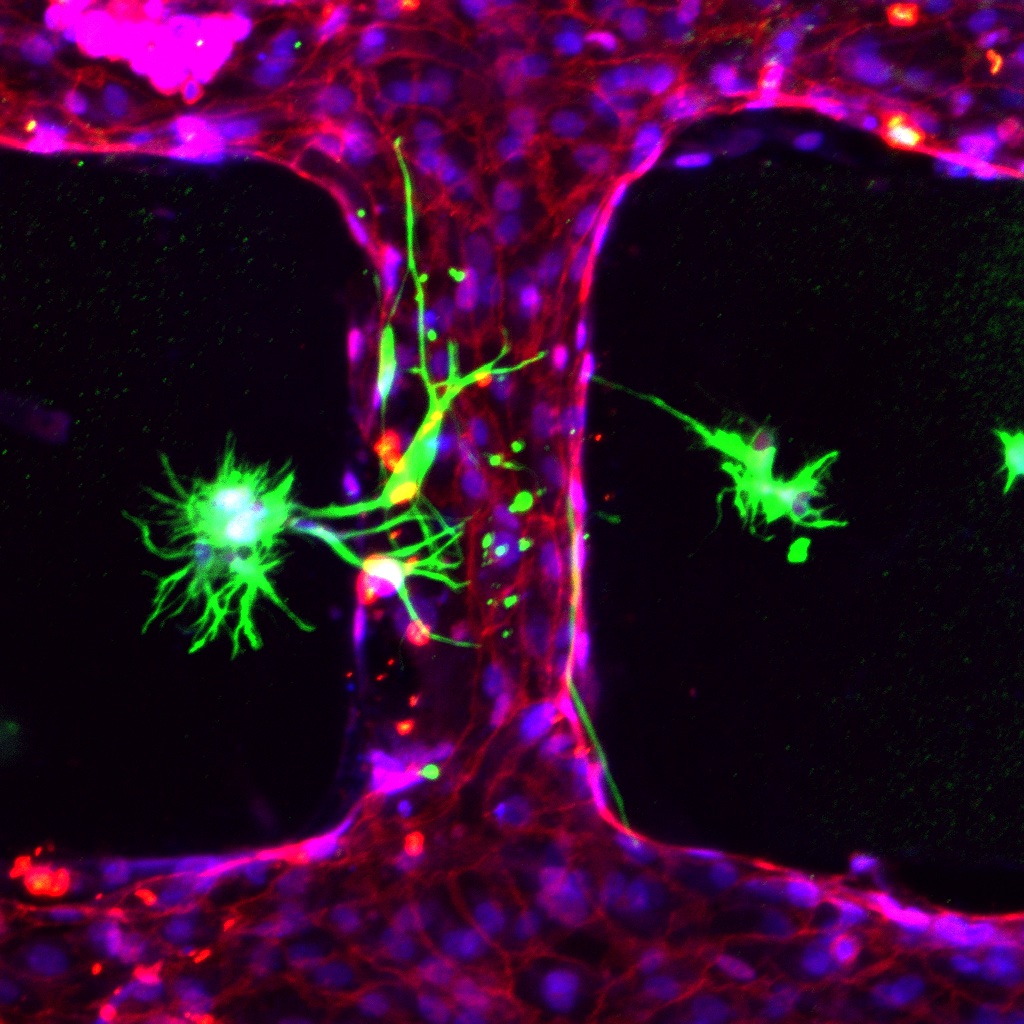
The COVID-19 pandemic has also shown us how important it is to understand the development of infectious diseases, and organoids and MPS devices can be incredibly helpful. During the pandemic and using both MPS devices and organoids, researchers across the world could replicate interactions between SARS-CoV-2 and organs like lungs, kidneys, or blood vessels. In addition, they could study immune responses and assess the effectiveness of therapies.
At EMBL Barcelona, the Haase and Bernabeu group joined forces to study how SARS-CoV-2, the novel coronavirus, produces microvascular inflammation.
“Results from this project can help us to identify how SARS-CoV-2 travels through the human body and whether or not existing drugs can reduce the impact of severe COVID-19, and may provide evidence for new targets to treat the disease,” said Marina Fortea, a joint postdoc in the Bernabeu and Haase groups.
In addition to studying diseases and performing drug screening and toxicity assays, organoids and MPS can be developed into assays used in personalised medicine. These technologies can be used to explore the effects of drugs in individual patients, using patient-derived tissue. Nowadays, researchers can generate successful organoids from almost every patient biopsy, and this holds great hope for personalised medicine.
Group Leader Talya Dayton generates lung organoids from patient-derived tumour tissue to study cancer. Her group also generates organoids from healthy tissue, and this allows them to compare healthy tissue and cancer tissue.
“The fact that we can generate organoids from healthy cells and tumour cells in the same defined in vitro system means that we can grow and study cancers across the entire existing range of malignancy,” said Dayton. According to her, while traditional 2D cell lines can usually only be grown from highly aggressive cancers, organoids can be grown from even very early cancers, and this allows scientists to use this system to study how early cancers can become more malignant and highly aggressive.
“In our lab, we use organoids and genetic engineering to recapitulate the transition from healthy cell to early cancer cell to highly aggressive cancer that is the basis of cancer formation and progression. Using this, we can try to predict therapeutic strategies that can either prevent this transition from happening or that can reverse it,” added Dayton.
At their heart, organoids and MPS devices are simplified three-dimensional in vitro structures that mimic organ and tissue function. They can be created using stem cells, patient cells, or tissue samples and can be designed to simulate human physiology better than traditional 2D cell culture. In the case of MPS, these devices can mimic critical aspects of organs such as mechanical cues, e.g. blood flow, tissue stretch, or hydrostatic pressure.
“Although 2D cell culture systems offer a high degree of flexibility and reproducibility, they do not fully capture the 3D complexity that cells experience in vivo – thereby limiting their ability to recapitulate tissue and organ function,” said Akinola Akinbote, PhD student at the Haase Group. “In comparison, MPS have the potential to better approximate the in vivo environment by recapitulating multicellular 3D arrangement and the extracellular microenvironments in vitro,”
Akinbote’s project seeks to harness human induced pluripotent stem cell technologies and microfluidics to generate perfusable cardiac-specific vascularised tissues – essentially mimicking human heart vessels on a matchbox-sized device. “Our objective is to establish a representative model that enables the investigation of the coronary microvessels form and function in vitro,” said Akinbote.
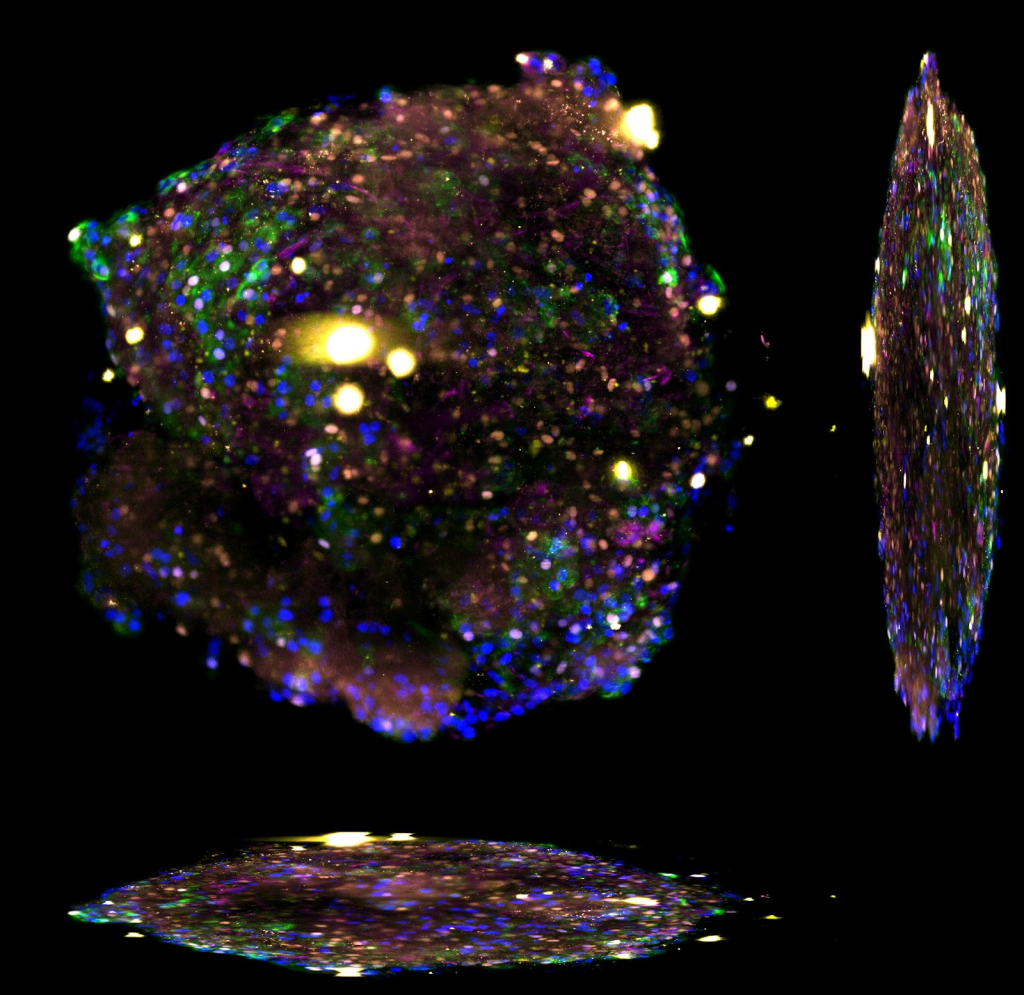
The Ebisuya group studies how and why we humans are different from other species when it comes to embryonic development. To study this, Miki Ebisuya and her group work with organoids of different species and compare them. The researchers are already working on several mammalian species, including rabbits, cattle, and rhinoceroses, setting up a ‘stem cell zoo’ in the lab.
“Our next project will focus on creating somitoids, from different species. Somitoids are organoids that mimic the precursor structures that give rise to the spinal column form during human embryonic development. We would like to measure the cell proliferation and cell migration speed of somitoids from different mammals to establish what and how somitogenesis is different among species,” said Ebisuya.
MPS devices differ in size and design. Ranging from approximately the size of a USB memory stick to that of a credit card, they vary depending on need and usage. At EMBL Barcelona, one example that utilises such flexibility is the placenta-on-chip system developed by Marta Cherubini, postdoc in the Haase Group.
“We use a small device made from a flexible polymer with a central port where we grow cells to create a placental barrier,” said Cherubini. “With the right nutrients and environment, cells spontaneously form vessels inside the chip. Around that port, there are several channels that allow us to supply fluids and molecules to the cells and analyse fetal-like vascular development.”
A critical aspect of performing successful research with MPS is to be able to develop your own devices, tailored to the needs of the project. EMBL Barcelona has led a project on campus to be able to fabricate such tools – a ‘maker-space’ or collaborative place, with equipment to design and produce tools, from macro to micro scales. This space is called the micro Fabrication Laboratory – µFabLab – and is now open for the whole PRBB community.
“The advantage of using laser cutters, 3D printers, and CNC milling machines is that you can design small parts and generally fabricate them in an afternoon,” said Kristina Haase, Group Leader at EMBL Barcelona and one of the main forces behind the creation of the µFabLab. “This is a huge advantage of prototyping – being able to make several iterations of a design quickly to optimise it for your needs and get it rapidly to the lab for biological experiments.”
Over the last 20 years, researchers have developed several types of organoids with strong resemblance to in vivo organs. However, organoid generation can be a lengthy process in some cases, and has relatively low throughput. The challenges of this field are somewhat related to the complexity of biology itself.
Building an MPS model presents the first challenge. Researchers need to capture the level of biological complexity and accuracy of the organ, as well as its size and its fluid volume. In organoids and MPS models, there are several types of cells that coexist in the same space. Creating and maintaining such cell heterogeneity is a challenge. At EMBL Barcelona, many MPS models include vasculature, which is a dynamic and complex system. In addition, obtaining sufficient cells to perform experiments is not always a straightforward process, especially if these are patient-derived.
In spite of the above-mentioned challenges, these 3D miniaturised organs and devices present many benefits: they provide researchers with greater insights into human physiology, they can better approximate the in vivo environment of organs, and they can be created with patient-derived tissue.
Recently, the US Food and Drug Administration (FDA) approved the use of microfluidic devices for drug testing, ending a federal mandate from 1938 that obliged experimental drugs to be tested on animals before they were used in human clinical trials. This new law opens the door to new technologies that aim to reproduce human physiology in vitro as a means of effective preclinical research.
There is also growing recognition from industry that these techniques are suitable for preclinical drug screening, tissue regeneration, and toxicology/safety evaluation. Advances in this field, including those led by EMBL Barcelona, will therefore represent a reduction in animal testing, more targeted drug evaluation, and the development of personalised medicine.

First published in 1999 as a black-and-white printed newsletter for EMBL staff and alumni, EMBLetc. has undergone many transformations in its 24 years of existence.

EMBLetc., the online magazine of Europe’s life sciences laboratory, celebrates its 24th birthday with its 100th issue. We took a walk through the past issues of this dynamic publication, and here are 10 recurring themes that emerged.

Click here to download a quick overview of all the articles included in this digital issue of EMBLetc.