Picture Release: More than meets the eye
‘Transformer’ protein makes different sized transport pods
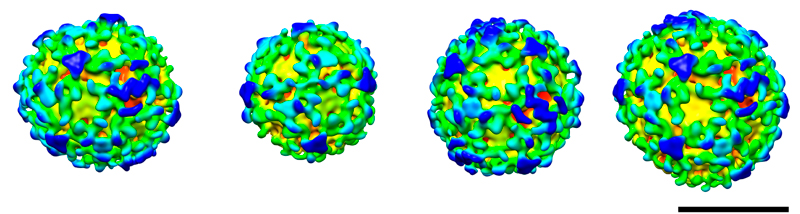
These spheres may look almost identical, but subtle differences between them revealed a molecular version of the robots from Transformers. Each sphere is a vesicle, a pod that cells use to transport materials between different compartments. The images, produced by Marco Faini from John Briggs’ lab at the European Molecular Biology Laboratory (EMBL), in Heidelberg, Germany, are the first high-resolution view of the three-dimensional structure of such a pod. They show a particular type of vesicle that is encased by a protein called COPI, and whose structure had never been seen before.
Several copies of the COPI protein attach to each other to form a coat around the vesicle’s membrane. Briggs and colleagues were surprised to find that the COPI building blocks are capable of a ‘transformer’ act: they can change shape to connect to more or fewer copies of themselves. So by changing the shape of individual COPI blocks, the cell could create vesicles of different shapes and sizes, for instance to transport different kinds of cargo.
Previously, scientists had been able to create and determine the structure of ‘cages’ formed by parts of the protein coats that encase other types of vesicles, but this study, published online today in Science, was the first to obtain high-resolution images of complete vesicles, budded from a membrane.
The work was carried out in collaboration with the lab of Felix Wieland at Heidelberg University in Germany.
Additional resources

Video: click image to play.
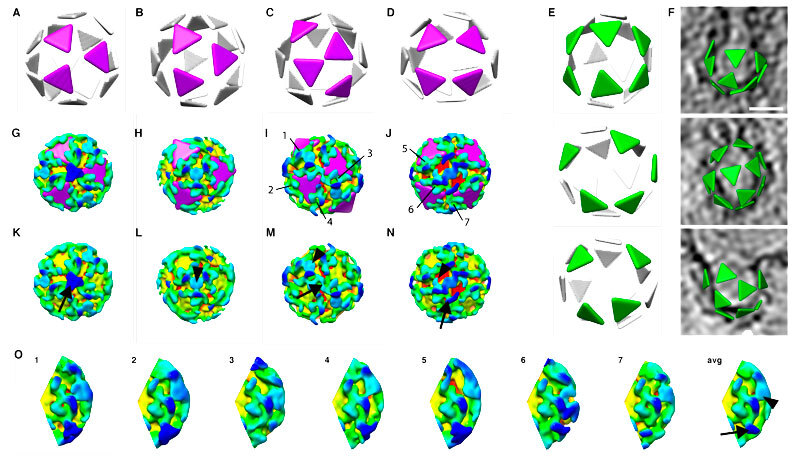
Building block: a COPI protein (yellow, green, blue) on the vesicle’s membrane (red) can change shape to connect to more or fewer copies of itself, making vesicles of different sizes and shapes.
Click to enlarge.

Video: click image to play.
Clicking together: depicting each copy of COPI as a triangle, the different arrangements of these building blocks become easier to spot.
Image and video credits: EMBL/M.Faini
Source article
Faini, M., Prinz, S., Beck, R., Schorb, M., Riches, J.D., Bacia, K., Brügger, B., Wieland, F.T. & Briggs, J.A.G. The Structures of COPI-Coated Vesicles Reveal Alternate Coatomer Conformations and Interactions. Published online in Science Express, 24 May 2012.
Article abstract
Transport between compartments of eukaryotic cells is mediated by coated vesicles. The archetypal protein coats COPI, COPII and clathrin are conserved from yeast to human. Structural studies of COPII and clathrin coats assembled in vitro without membranes suggest that coat components assemble regular cages with the same set of interactions between components. Detailed 3D structures of coated membrane vesicles have not been obtained. Here, we solved the structures of individual COPI-coated membrane vesicles by cryo-electron tomography and subtomogram averaging of in vitro reconstituted budding reactions. The coat protein complex, coatomer, was observed to adopt alternative conformations to change the number of other coatomers with which it interacts, and form vesicles with variable sizes and shapes. This represents a fundamentally different basis for vesicle coat assembly.



