Structural basis of tRNA recognition by the m3C RNA methyltransferase METTL6 in complex with SerRS seryl-tRNA synthetase
Nature Structural & Molecular Biology 25 June 2024
10.1038/s41594-024-01341-3
The Kowalinski Group at EMBL Grenoble has revealed how one enzyme hitches a ride on another to recognise tRNA
By Eva Kowalinski, Group Leader, EMBL Grenoble
Imagine your body as a highly organised factory where workers tirelessly assemble proteins around the clock. These proteins are the machines and scaffolds that make up your body and are essential for various functions. In this factory, special delivery trucks called transfer RNA (tRNA) deliver amino acids – the crucial building blocks of proteins – to the protein-making machinery – ribosomes.
The Kowalinski group at EMBL Grenoble has here used a combination of structural biology methods to shed new light on the process that ensures that these tRNA delivery trucks are optimised for their tasks.
The team studied tRNA modification enzymes – a type of specialised molecular workers, which can ‘customise’ these tRNA delivery trucks. They make specific changes or additions to the tRNA structure, which enhance the efficiency and accuracy of the protein-building process. This ensures that the tRNA trucks are optimised and tailored for their respective tasks, leading to a more reliable and precise production of proteins.
Given that all tRNA molecules look very similar, but the tRNA modification enzymes only work with specific types of tRNA, the question arises: how do the modification enzymes precisely select specific tRNA molecules to modify, and ensure they don’t mistakenly choose the wrong ones?
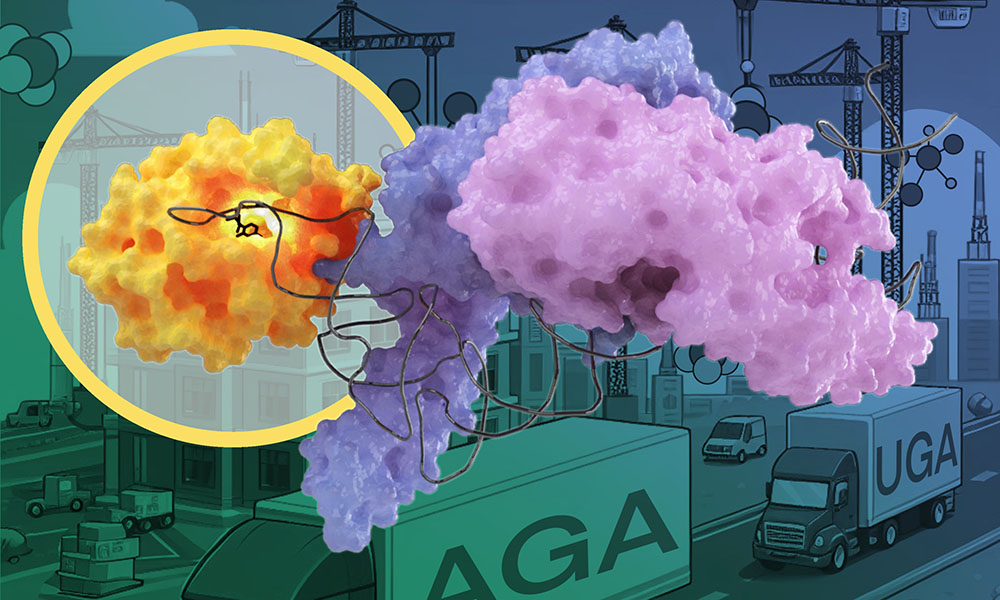
To answer this, the Kowalinski group carried out experiments that have now revealed how one such tRNA modification enzyme, called METTL6, picks its specific tRNA target.
When scientists want to see the tiny details of proteins, they can use a powerful technique called cryo-electron microscopy. Here, scientists first rapidly freeze the protein, helping to capture the protein in its natural 3D shape without any distortions. Next, they use a beam of electrons, which creates – like a spotlight – shadows that reflect the 3D structure of the protein.
“We use these shadows to compute the shape and structure of the protein,” said Luciano Dolce, a postdoc involved in the study. “We used this technique to reveal the structure of METTL6 together with its target tRNA.”
In the case of the METTL6 tRNA modification enzyme, the researchers figured out that it does not act on its own, but interacts with another enzyme – a ‘tRNA synthetase’.
In the analogy above, tRNA synthetases are the workers responsible for loading the tRNA delivery vehicles and ensuring that the right amino acids are loaded onto these trucks. Each tRNA delivery truck carries a specific code or pattern that matches with a code on the construction site. tRNA synthetases are very smart enzymes that can read the nucleotide code of the tRNA trucks and then find and load the correct amino acid that matches the code.
The scientists found that the tRNA modification enzyme METTL6 on its own is not particularly specific and not very efficient at doing its job. Instead, METTL6 takes the hand of its smart friend – the serine tRNA synthetase. This tRNA synthetase specifically binds tRNAs that carry the code for an amino acid called serine.
When the serine tRNA is bound to the serine tRNA synthetase enzyme, it is much easier to distinguish from other tRNAs. You could think of serine tRNA synthetase as a very smart friend that helps METTL6 figure out which tRNA to modify. The authors of the study believe this friendship is the first known example of a tRNA-modifying enzyme using a tRNA synthetase as a recognition factor.
This discovery is more than just figuring out the structure of the METTL6–serine tRNA synthetase complex bound to tRNA; it’s like discovering a powerful new tool for making better medicines. This is particularly important since METTL6 is highly abundant in tumour samples of cancer patients, e.g. in some breast and liver cancers.
Studies in cell cultures and mice suggest that slowing METTL6 down might help reduce cancer growth. The new findings by the Kowalinski Group show how METTL6 works and how it recognises tRNA. This will enable designing precise drugs to slow down tumour growth, which may become a smarter strategy in the ongoing battle against illnesses – one that comes from understanding the inner workings of the body’s molecular machinery.
Nature Structural & Molecular Biology 25 June 2024
10.1038/s41594-024-01341-3