Early autonomous patterning of the anteroposterior axis in gastruloids
Development 11/2024
10.1242/dev.202171
Researchers from the Trivedi Group at EMBL Barcelona unveil how cells can behave autonomously during early development
We all start our lives as symmetric balls of cells. In humans, during the first few weeks after fertilisation, embryonic cells undergo several rounds of division, increasing their mass. Then comes gastrulation, the process that changes everything and establishes our body plan. During gastrulation, the collection of uniform cells that make up the early embryo break symmetry and reorganise into a multi-layered structure with distinct cell types. At this pivotal moment, our body plan is set. Gastrulation also establishes the three body axes: head–tail, front–back, and left–right. This process requires cells to interact and coordinate with each other with astonishing precision. However, how this is achieved is still largely a mystery.
The Trivedi Group at EMBL Barcelona studies how cells give rise to our body plan and has now published a study in the journal Development that may enhance our understanding of early mammalian development.
“It is believed that the anterior–posterior axis of our body, that means our head-to-feet structure, requires external signals to be developed. In our study, we show that cells can independently orchestrate the first steps of symmetry breaking, without any external input,” said Vikas Trivedi, Group Leader at EMBL Barcelona.
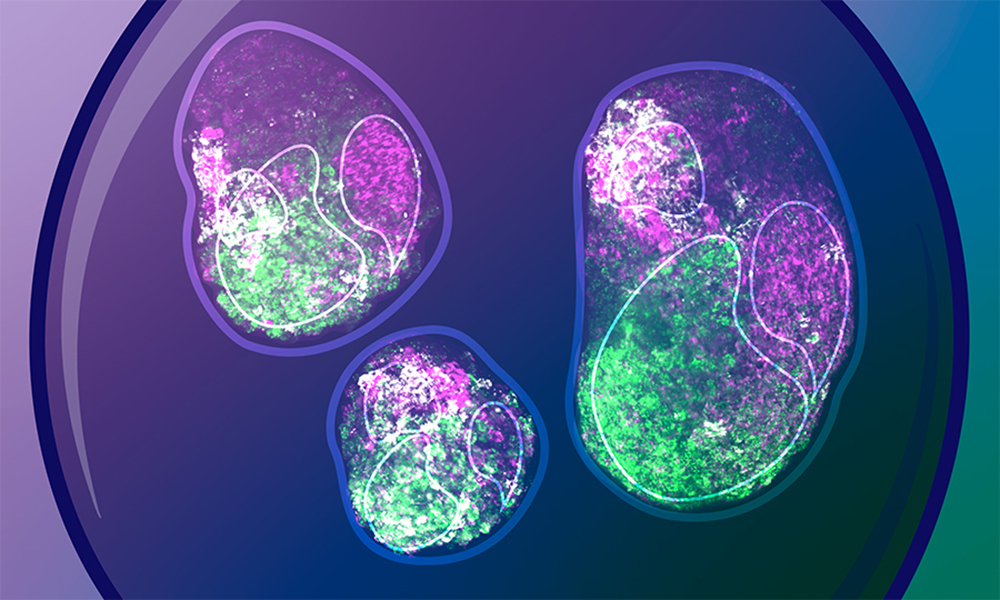
Gastruloids are three-dimensional aggregates of embryonic stem cells grown in the lab in a controlled environment. Over time, they form structures that mimic many of the features of early mammalian embryos, thus allowing us to peek into developmental processes that would be almost impossible to examine inside the uterus. But what is most remarkable about these little cell aggregates is that they manage to form such structures without the patterns or cues that typically instruct embryonic cells within the uterus. Thus, they can help us understand the true potential of embryonic cells to self-organise while creating a body plan.
“Our work aims to detail the earliest developmental events in gastruloids by culturing them under minimal conditions,” said Kerim Anlas, former PhD student at Trivedi Group and co-first author of the work. “This could reveal previously unknown features of early embryogenesis that cannot be demonstrated from studying only the native embryo.”

The Trivedi team grew gastruloids from mouse embryonic stem cells for the first 72 hours after fertilisation, the time window when mouse embryonic cells make the most important decisions to define a head–tail axis. At different time points, they analysed gene expression and other molecular signatures in individual gastruloid cells.
The team found that gastruloids can break symmetry, with cells at two ends of the structure attaining distinct properties (a process known as polarisation), without external chemical stimulation. For example, a subset of gastruloid cells begin to express a gene called Brachyury and undergo changes in cell shape, gene expression, and motility. These would eventually form the mesoderm – the embryonic tissue layer which later gives rise to muscles and bones. Other specialised cell types also emerge, expressing specific markers and occupying distinct locations within the gastruloid. These are the first steps in anterior–posterior axis formation, and as the scientists showed, can happen in the absence of external signals.
Notably, the researchers also explored how similar cells in gastruloids are to those in embryos. They observed that while in the initial stages, gastruloid and embryonic cells express different genes, they nevertheless give rise to very similar differentiated cell types. “This suggests that cells can reach similar end states both in gastruloids and in vivo but with distinct initial states,” said Trivedi.
Nicola Gritti, Image Analysis Specialist at the Mesoscopic Imaging Facility, former postdoc at the Trivedi Group, and co-first author, believes that these findings can serve as a new way of studying early development in mammals. “Studies on embryo-like in vitro systems commonly aim to present a faithful replica of native development and hence focus on highlighting similarities to the actual embryo,” he said. “Here, we report key differences to the events observed in vivo that have not yet been thoroughly studied.”
The Trivedi Group aims to further study the alternative developmental pathways observed in gastruloids, and combine in vitro systems with engineering, data science and theoretical models to understand the versatility of stem cells.
Development 11/2024
10.1242/dev.202171