MFSD1 with its accessory subunit GLMP functions as a general dipeptide uniporter in lysosomes
Nature Cell Biology 5 June 2024
10.1038/s41556-024-01436-5
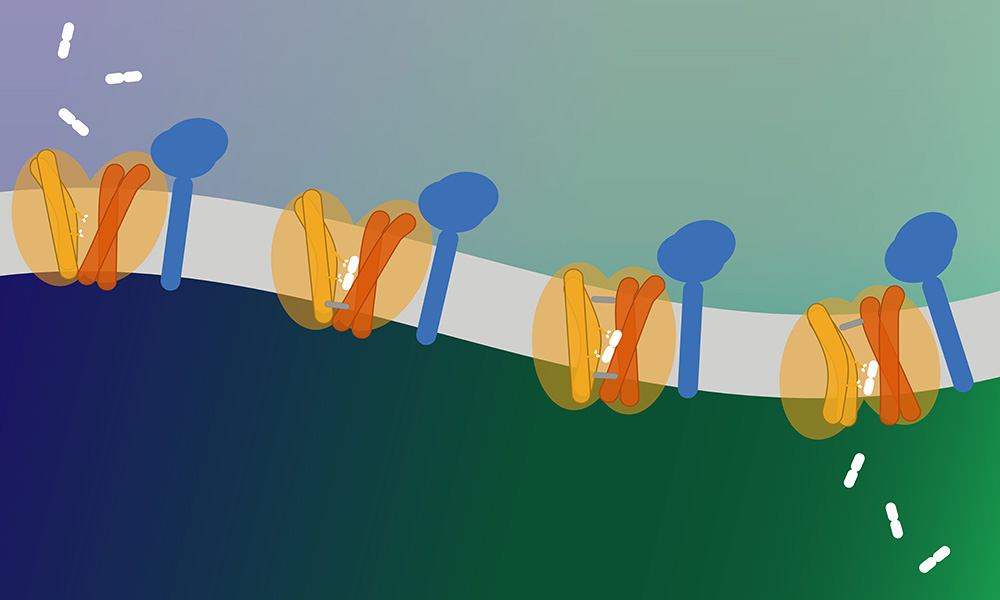
The groups of Christian Löw (CSSB, EMBL Hamburg), Markus Damme (Christian-Albrechts-University Kiel), and Bruno Gasnier (CNRS and Université Paris Cité) worked together to reveal the structure and function of a previously unknown lysosome transporter. Their findings have recently been published in Nature Cell Biology.
Lysosomes are organelles that function as a waste disposal and recycling system within the cell. They break down larger macromolecules such as proteins and lipids into smaller, lighter compounds like amino acids, monosaccharides, or fatty acids. These smaller compounds, known as metabolites, are then transported into the cell’s cytoplasm.
The Damme group at Christian-Albrechts-University Kiel seeks to elucidate the function of specific proteins on the lysosome’s membrane.
“Several years ago, we noticed that the Glycosylated Lysosomal Membrane Protein (GLMP) binds tightly with an orphan transporter named Major Facilitator Superfamily Domain Containing 1 (MFSD1),” noted Markus Damme, one of the study’s three corresponding authors. As the term ‘orphan transporter’ indicates, MSFD1’s function and substrate were unknown.
To help reveal MFSD1’s function, Damme reached out to the Löw group at the Centre for Structural Systems Biology (CSSB) and EMBL Hamburg. The Löw Group’s research focuses on understanding peptide transporters known as POTs (proton-coupled oligopeptide transporters).
“I was intrigued by MFSD1 and wanted to help figure out its role in the lysosome,” explained Löw, currently a visiting group leader at EMBL Hamburg and another of the study’s corresponding authors. “I was also confident that the different technologies and methodologies available to us at CSSB would be essential in helping us unravel this mystery.”
The work was supported in part by the EMBL Sample Preparation and Characterisation Facility, Protein Production Core Facility and CSSB’s cryo-EM multi-user facility on-site.
Using a combination of techniques, including fluorescence spectroscopy and differential scanning fluorimetry (nanoDSF), the researchers discovered that MFSD1 not only binds but also transports dipeptides, peptides comprised of just two amino acids. The researchers were then able to show how the MFSD1/GLMP complex binds to dipeptides.
“We were able to determine the complex’s structure in an outward-open conformation,” explained Katharina Jungnickel, an EMBL EIPOD Fellow and one of the first authors of the paper. “We additionally saw density of the dipeptide at the binding site. Together with molecular dynamics simulations (by Reza Mehdipour, Ghent University), we verified that dipeptide binding is mainly facilitated by the coordination of its N- and C-termini.”
The Gasnier group at the Université Paris Cité performed some clever experiments which enabled the researchers to discover the mechanism used by MFSD1 to transport the dipeptides.
“We discovered that MFSD1 is a passive uniporter that only transports dipeptides along its own gradient,” stated Bruno Gasnier, the paper’s third corresponding author. “This led us to develop a new assay which revealed that MFSD1 transports a much wider spectrum of dipeptides than initially thought.”
The insights gained by the researchers indicate that MFSD1 provides an alternative route to supply amino acids for biosynthetic pathways when other lysosomal amino acid exporters are overloaded.
“This was an amazing collaborative effort which combined labs with different expertise that were driven by the need to answer biological questions,” noted Löw. “I am looking forward to finding out more about MFSD1 and its overall role in nutrition sensing.”
This article has been adapted from a news article by the Centre for Structural Systems Biology (CSSB). Find the original story at the CSSB website here.
Nature Cell Biology 5 June 2024
10.1038/s41556-024-01436-5