
Taking biological imaging to the next level
Extraordinary funding from the Chan Zuckerberg Initiative supports two large collaborative projects that will boost imaging technology development
By Stephanie Alexander, EMBL Imaging Centre Research & Service Coordinator
Developing advanced imaging technologies is critical to advancing life sciences research. It is especially important to explore the detailed structure and function of tissues, cells, and macromolecules in ways previously unimaginable, offering unprecedented insights into both healthy and diseased states.
The Chan Zuckerberg Initiative (CZI) recently funded two multidisciplinary projects across EMBL, coordinated by the EMBL Imaging Centre, to support the development of two such emerging technologies. These are: the integration of 3D X-ray imaging of tissues with spatial transcriptomics, and the development of cutting-edge cryo-electron microscopy techniques for multiscale imaging, down to the level of macromolecular complexes inside cells.
Developing advanced imaging to transform precision medicine
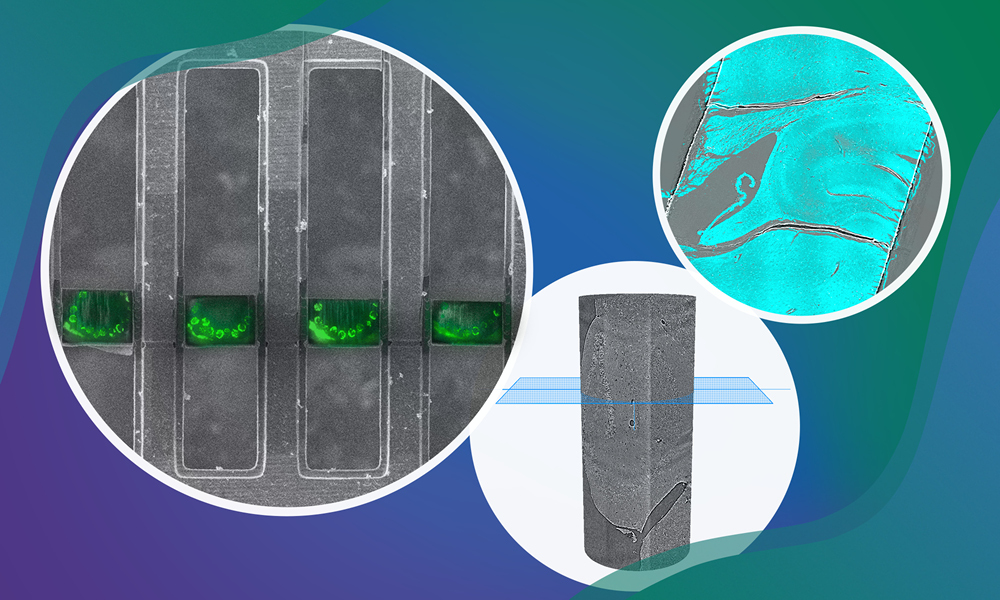
The first funded project aims to develop HiTT2T – high-throughput X-ray tomography to targeted transcriptomics. This is a pipeline that integrates 3D X-ray imaging with spatial transcriptomics, aiming to advance precision medicine.
Currently, high-throughput X-ray tomography at EMBL Hamburg provides rapid, high-resolution 3D imaging of entire unstained tissue biopsies, while EMBL Rome’s spatial-omics service platform within the Light Imaging Facility enables multiplexed RNA detection, commonly known as targeted transcriptomics. Combining these technologies and supported by the Data Science Centre to generate new computational tools to analyse this type of data, HiTT2T will facilitate the targeted molecular analysis of tissue samples, revealing cellular heterogeneity in health and disease more effectively than traditional 2D methods.
This project is led by Elizabeth Duke, who heads the Biological X-ray Imaging Team at EMBL Hamburg. “Such an integrated workflow promises to transform diagnostics and personalised medicine by delivering detailed molecular insights for every patient biopsy, improving disease understanding and treatment strategies,” she said.
“A long-standing goal in imaging of biological tissues is to link the detailed cellular morphology of a tissue with its repertoire of macromolecular constituents,” said Alvaro Crevenna, Head of the Light Imaging Facility at EMBL Rome. “Such a link would enable a molecular understanding of cellular and subcellular heterogeneity in healthy and pathological tissues.”
This project entails advanced multi-modal big image data handling and analysis, which is supported by the Bioimage Analysis Support Team (BAST) of EMBL’s Data Science Centre. “Standardised analysis workflows for tissue imaging and molecular profiling, along with automation in data integration tools, ensure reproducible results,” said Christian Tischer, BAST Team Leader.
Cryo-electron microscopy across scales
Similarly, cryo-electron tomography and cryo-focused ion beam (FIB)-scanning electron microscopy (SEM) are high-resolution imaging technologies that are transforming structural cell biology. “These breakthrough technologies allow us to study the 3D architecture of organelles and molecular structures in unperturbed cells,” explained Simone Mattei, electron microscopy Team Leader at the EMBL Imaging Centre. “This ultimately provides insights into cellular processes, disease mechanisms, and drug interactions.”
To advance these capabilities, the second funded project focuses on establishing plasma-FIB technology at EMBL Heidelberg as a superior alternative to the more routine Gallium-FIB milling, especially for complex multicellular samples, along with automated software for high-throughput, minimally damaging cryogenic specimen preparation and contextual 3D FIB-SEM imaging. “These innovations will enhance the analysis of tissue biopsies and in vitro disease models, with potential applications for understanding disease mechanisms and eventually even in precise diagnostics through automated data processing and annotation,” said Julia Mahamid, who is Head of EMBL’s Molecular Systems Biology Unit and leading this project together with Mattei.
Besides Mattei and Mahamid, Anna Kreshuk, Group Leader at EMBL Heidelberg and Yannick Schwab, Team Leader and Head of Electron Microscopy Core Facility, are also involved in the project.
By improving accessibility and ease of use, both projects aim to accelerate discoveries in life sciences and promote the widespread adoption of imaging technologies in structural, cell, and multicellular biology.
“These awards will hopefully be the basis of a long-term collaboration between the EMBL Imaging Centre and the new Chan Zuckerberg Institute for Advanced Biological Imaging in the context of imaging technology development and early open access services for the scientific community,” said Jan Ellenberg, Head of the EMBL Imaging Centre.


