Sense of space
Like the stone in a fruit, nucleus size scales with cell size – in healthy cells at least – so that the nucleus can’t take up the whole space inside the cell. However, the underlying molecular mechanisms behind that controlled growth have never been fully understood.
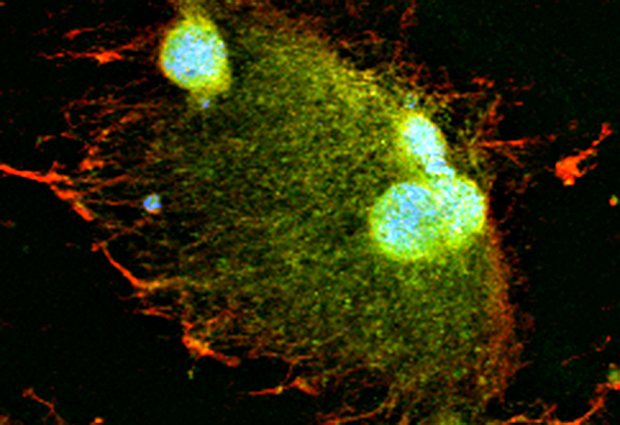
Scientists at EMBL Heidelberg demonstrate in Developmental Cell that the space surrounding a growing nucleus – not the overall volume of the cell – is also a crucial factor in regulating how fast the nucleus grows. They also show that the motor protein dynein and so called ‘microtubules’ which are part of the cytoskeleton are involved in this process.
“Although it was known that the nucleus size correlates with the space surrounding it as well as with volume of the cell, nobody knew exactly how the nucleus was able to sense what space was available,” said Yuki Hara from EMBL’s Genome Biology Unit. “One theory was that the nucleus simply grew until all the available material was used up – but this does not explain how spatial constraints affect nucleus size. To answer this question, we designed micro-devices that allowed us to mimic a variety of space constraints inside a cell so we could systematically analyse the effects on how nuclear size is controlled.”
We designed micro-devices that allowed us to mimic a variety of space constraints inside a cell.
The researchers used extracts from frog eggs that would grow nuclei in vitro and introduced these into the devices which were carved with channels of different micro-scale sizes to mimic different-sized spaces within a cell.
They found that the nuclei grew slower when there was less space around them. Furthermore, when additional materials known to be necessary for nuclear growth were added into micro-devices, the speed of growth increased – even within the smaller space, which means that the recruitment of materials to the growing nucleus was the dominant factor in controlling nuclear size.
The team then looked in more detail at what was happening within the space around the nucleus and found that the speed of nuclear growth was relative to how large an area around the nucleus was occupied by the microtubules. Microtubules – cellular components along which materials needed for nuclear growth are transported – are known to work together with dynein, the motor protein that enables that transportation. When the function of the microtubules was inhibited, the speed of nuclear growth was much slower. Similarly, when the function of motor protein dynein was blocked, the nuclear growth speed also remained slow. The results were replicated in frog embryos to confirm the same results in vivo.
When the space around the nucleus is constrained, this restricts the expansion of microtubules, resulting in fewer materials available to the nucleus.
“When the space around the nucleus is constrained, this restricts the expansion of microtubules, resulting in fewer materials available to the nucleus which then grows more slowly, resulting in smaller nuclei for any given time point. This worked conversely too, so where the nucleus was less confined, the microtubule-occupied space could fully expand and so more materials were recruited by the nucleus and it grew faster and will reach a larger size,” said co-researcher Christoph Merten who heads the microfluidics lab in which the work was carried-out.
These results suggest that the position of the nucleus inside the cell in vivo is critical. If the nucleus is too close to the plasma membrane, there is not enough room around it for the microtubule-occupied space to expand fully, and the nucleus will grow more slowly and remain smaller in size. However, if the nucleus sits in the centre of the cell it has more space around it and can grow faster. The researchers believe this might provide a further explanation for why in most cells, the nucleus tends to be well centred.



