Go with the flow
Looking for a needle in a haystack is hard enough. But now imagine that the haystack fills an entire room, it’s dark and the pieces of straw and the needle are less than half the width of a human hair.
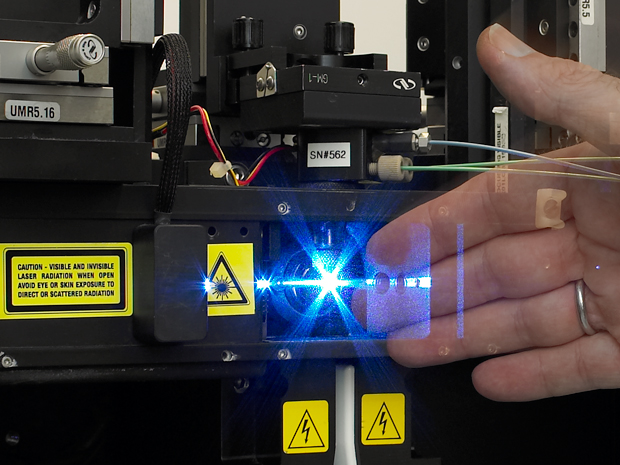
Worse, you have to sort through the whole lot before lunchtime armed only with a torch. Such are the challenges faced by Alexis Perez Gonzalez, manager of the EMBL Flow Cytometry Core Facility at EMBL Heidelberg, and his colleague Daniel Bilbao, of the EMBL Monterotondo facility.
The ‘haystacks’ in question are samples of cells, the trillions of tiny units that form our bodies and those of other creatures. The ‘needles’ are very rare cells – perhaps as scarce as one in a billion. Sometimes Perez Gonzalez and Bilbao also have to discriminate between varying kinds of needles, whose differences are barely detectable. Their expertise is helping EMBL researchers push back the boundaries of fields ranging from the molecular genetics of cancer to neurobiology. “With every researcher and almost every project, we have to come up with new solutions to address those challenging questions,” says Bilbao. “Every day there is something new.”
The secret of their success is a technique called flow cytometry, which allows them to sort through cell samples with extraordinary speed and accuracy. “You can easily go through millions of cells in seconds,” says Perez Gonzalez. This allows the team to screen enough samples to find cells that would be too rare to detect using a microscope.
A glow in the flow
As their name suggests, flow cytometers analyse streams of liquid containing cells. These cells are grown in lab dishes or extracted from samples such as blood samples. They are mixed with liquid and loaded into the machine, which then streams a flow of cells past a beam of intense light in the form of a laser. To make the cells visible, researchers attach fluorescent dyes to them. The dye glows when it meets the laser light, allowing the machine to capture a snapshot of it.
As long as we have a fluorescent dye, we can do it. And if we don’t have one, we look for it.
Some dyes are attached to molecules called antibodies that home in on specific proteins in or on a cell; others rely on different biochemical tricks to tag particular molecules. Whatever the strategy, the dye allows researchers to identify cells that contain particular proteins of interest – perhaps one that marks out a cell as belonging to a specific cell type, such as a white blood cell. As well as finding their target cells, researchers can also use flow cytometry to look for changes in the kinds or amounts of proteins (or other molecules) present in those cells. Scientists can use several different-coloured dyes to tag several different proteins simultaneously. What’s more, they can also measure other properties of cells: whether a cell is dead or about to die, stressed or happily proliferating, for example. “As long as we have a fluorescent dye that reflects the property that we want to measure, we can do it. And if we don’t have a dye, we look for it,” says Bilbao.
The cytometer can analyse the glow pattern of each sample to determine how many kinds of each cell it contains, or, in the case of a method known as fluorescence-activated cell sorting, pluck cells from the stream and sort them into different containers. The technique is constantly finding new applications in all fields of cell and molecular biology. “Essentially, the list is endless,” says Perez Gonzalez.
Endless applications
Eileen Furlong’s team at EMBL Heidelberg, for example, wanted to study how sections of DNA called enhancers control the early development of fruit fly embryos. This involved finding rare cells in the fly embryos whose nuclei had the correct properties for study. “The issue with this project was the scale of it,” says Perez Gonzalez. Furlong’s team needed to find 40 million target nuclei per batch. Thanks to their close collaboration with Perez Gonzalez, they were eventually able to purify 100 million nuclei in half a day, adding up to several billion nuclei found so far.
Someone may come with a crazy idea that will end up in a Nature paper and you need to give full support to this idea
Furlong’s project was an example of needle-finding. Jan Ellenberg’s team, which studies cell division, presents Perez Gonzalez with an additional problem: identifying different needles even though they are hard to see. As well as being rare, the cells Ellenberg is looking for glow only very faintly. But the machines at EMBL Heidelberg contain unusually powerful lasers, meaning that even though each snapshot lasts only 2 millionths of a second, there is enough light for the detector to capture the glow.
The ability to quickly and precisely measure up to 20 different properties in a single cell has allowed the Monterotondo facility to uncover hitherto hidden diversity in the samples they test. For example, it is helping Paul Heppenstall’s group look for populations of neurons responsible for sensing pain and touch, and aiding Martin Jechlinger’s team’s search for stem cells involved in breast tumours.
These strong lasers, together with the ability to modify the cytometers, mean that Perez Gonzalez and Bilbao have the flexibility to adapt the instruments and propose new ways of using them to meet the ever-more demanding challenges their users bring. “Someone may come with a crazy idea that will end up in a Nature paper and you need to give full support to this idea,” says Perez Gonzalez. “This normally means challenging the instrument to its limits.”
How do you become a cytometrist?
It’s largely a matter of ‘learning by doing,’ says Perez Gonzalez: