From patients to the lab (and back)
Acute lymphoblastic leukaemia (ALL), the most common form of cancer in children, is a multi-faceted disease: various subtypes exist, some of which remain incurable. An international team of researchers, including scientists from EMBL, has identified key genetic specificities of the incurable subtype TCF3-HLF-positive ALL, as well a small molecule that has proven effective against the tumour cells in a drug screening assay.
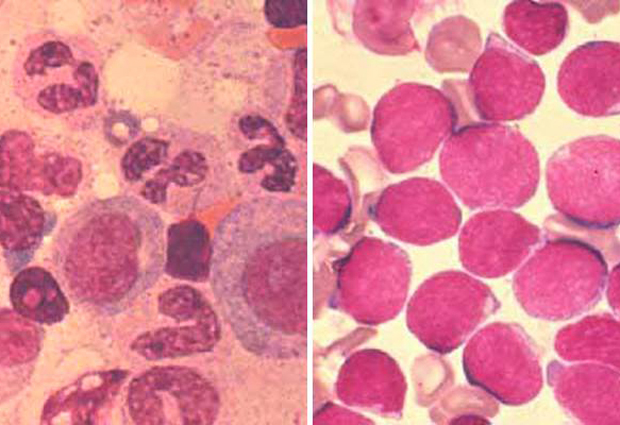
TCF3-HLF-positive ALL constitutes less than one per-cent of all paediatric ALL. Yet, its prognoses are dismal: relapse and death occur within two years of the diagnosis. By contrast, TCF3-PBX1-positive ALL is more common, but with much better chances of patient survival. Both disease types seem closely genetically related, but until now it was unclear why TCF3-HLF-positive ALL is unresponsive to common forms of therapy, even ones that work on TCF3-PBX1-positive ALL. An international research consortium, which brought together clinical and basic researchers from EMBL, Zürich University Hospital, Medizinische Hochschule Hannover, Heinrich-Heine Universität Düsseldorf, Christian-Albrechts-Universität Kiel, the Max-Planck Institute for Molecular Genetics, and others, decided to tackle that question.
As their names indicate, both types of ALL carry a mutation of the transcription factor TCF3. More precisely, TCF3 becomes fused with other genes – HLF and PBX1 respectively; however, this fusion alone doesn’t lead to leukaemia and the two types respond differently to treatments. So the scientists went on to look for other genetic specificities.
We wanted to identify any alterations that are specifically seen in the non-curable disease but not in the curable disease, and vice versa.
They sequenced and compared the genomes of both subtypes, using samples from ALL patients. “We wanted to identify any alterations that are specifically seen in the non-curable disease but not in the curable disease, and vice versa, so that we could learn what possible treatment mechanisms might have been overlooked and could be used in the future,” explains Stephanie Sungalee, research fellow in the Korbel group at EMBL who worked on the genomic analysis required for the study.
The researchers discovered that, in TCF3-HLF-positive ALL, another gene, called PAX5, is partly inactivated. This appears to result in overexpression of BCL2, a known oncogene favouring cell proliferation. As a consequence of this finding, the consortium identified venetoclax, a BCL-2 antagonist that is already being tested in other leukaemias, as a possible treatment option, which proved effective in drug screens with samples from patient cell cultures and cells isolated from a TCF3-HLF-positive xenograft mouse model.
We basically were able to go all the way from analysing genomic data to testing out new treatments in cell lines and mice.
No clinical trial has been conducted yet, so these results only provide clues on what a potential treatment could be. However, they fully testify to the immense potential of coordinated interdisciplinary and international research that connects clinics and patients directly to the lab. “What is so remarkable about this study is that through this interdisciplinary collaboration, we basically were able to go all the way from analysing genomic data to testing out new treatments in cell lines and mice that in the future may influence therapy in patients,” says Jan Korbel. “Combining all this expertise is very exciting and an efficient way to determine new practical solutions for the patients.”


