One gene, two proteins, one complex
"To see two proteins produced from the same gene that then bind together to form a complex – that is truly unique!” says EMBL Hamburg group leader Rob Meijers excitedly.
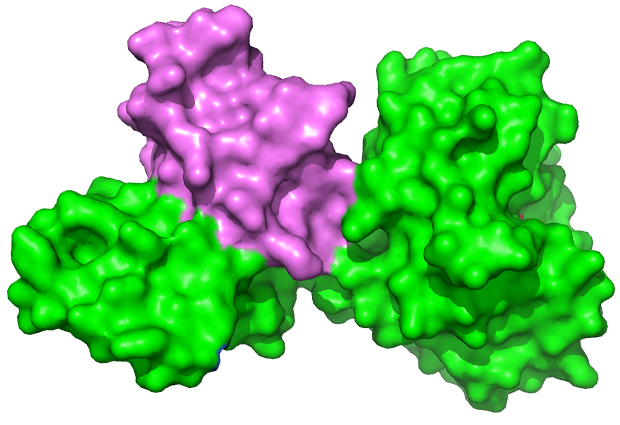
In a study recently published in the Journal of Biological Chemistry, Meijers and collaborators from the Institute of Food Research in Norwich, UK, show that the genes of viral enzymes that degrade the cell walls of Clostridia bacteria produce not the usual one, but two proteins. The results give insights into how these enzymes degrade the bacterial cell wall, and could be used to combat a range of Clostridium infections.
Dotted along the seemingly infinite string of A, T, G and C that make up an organism’s DNA, specific triplets of these letters – known as ‘codons’ – indicate where the cell’s machinery should start, and stop, when it translates the language of genes into proteins. Some genes have not one, but several start codons, resulting in proteins of different lengths being produced from one gene. “This study shows how two such proteins from the same gene form a complex, and how the shorter protein regulates the full length protein,” says Meijers, “to our knowledge that has never been seen before.”
Coming of phage
The double-packed gene studied by Meijers belongs to a virus that preys on species of Clostridia bacteria. After infecting their target, these viruses – or bacteriophages – break open the bacterial cell wall using enzymes known as endolysins, thereby destroying the bacterial cells. “It’s a perfectly timed process,” says Meijers, who is interested in understanding how these enzymes work. “At a fixed time point after infection, when all resources in the bacteria cell have been exhausted and the viral progeny are ready to be released, the endolysins start to break open the cell wall.” But just what triggers the endolysin to start its work is not yet understood.
Bacteriophages, and particularly their cell-wall degrading enzymes, are very interesting research objects.
With their precise choice of target, bacteriophages have long been considered potential allies in the fight against persistent bacterial infections. “In an era when many bacteria have evolved mechanisms to make them resistant to treatments such as antibiotics, we urgently need alternative ways of combatting bacterial infections,” explains Meijers. “Since they naturally destroy specific bacteria, bacteriophages, and particularly their cell-wall degrading enzymes, are very interesting research objects. How do they do it, and what can we learn from them?”
A cheesy problem
Of the approximately 100 species of Clostridium, a handful play a disruptive role in human society. Meijers and collaborators focused on C. tyrobutyricum, the byproducts of which in raw milk represent a significant economic drain for the cheese making industry. This study presents the 3D atomic structure of the complete C. tyrobutyricum endolysin bound to a smaller protein called Cell-wall Bound Domain, or CBD – the structure of which was solved by the group in 2014. “These two proteins are both coded for by the same gene,” says Meijers. Reading and translating the full-length gene produces the endolysin; reading from the second start codon produces the small CBD molecule.
It seems the bacteriophage uses both proteins to regulate the activity of the endolysin.
“It seems the bacteriophage uses both proteins to regulate the activity of the endolysin,” says Meijers. By removing the second start codon from the gene, the group shows that the activity of the endolysin is greatly reduced. Too much CBD, however, and activity is also slowed. These findings build on earlier work: “We originally thought that it was the endolysin complex that initiated and activated the bacteria cell wall break-up,” Meijers adds, “But actually it seems the activation of the endolysin begins in the gene itself.”
The long and short of it
After inserting the endolysin gene into milk bacteria used in cheese making, the researchers could show that both the full endolysin enzyme and the smaller CBD were produced by the new host. “We tricked the milk bacteria into producing the endolysin and the CBD for us, but these proteins remain trapped inside the bacterial cell,” explains Meijers. “We have made the full-length endolysin pass across the cell wall, but so far not the shorter CBD – without both parts, no complex forms and activity is reduced.” This highlights again how important both parts are, and presents a potential path for future molecule design for treating Clostridia infections. Meijers concludes: “Cheese makers currently add large amounts of enzymes to the cheese to combat the effects of the Clostridia – maybe in future we can just use small amounts of modified milk bacteria that will effectively destroy any Clostridia present.”