Structure of HIV capsid within virus visualised
EMBL scientists use new techniques to describe the architecture of conical HIV capsids
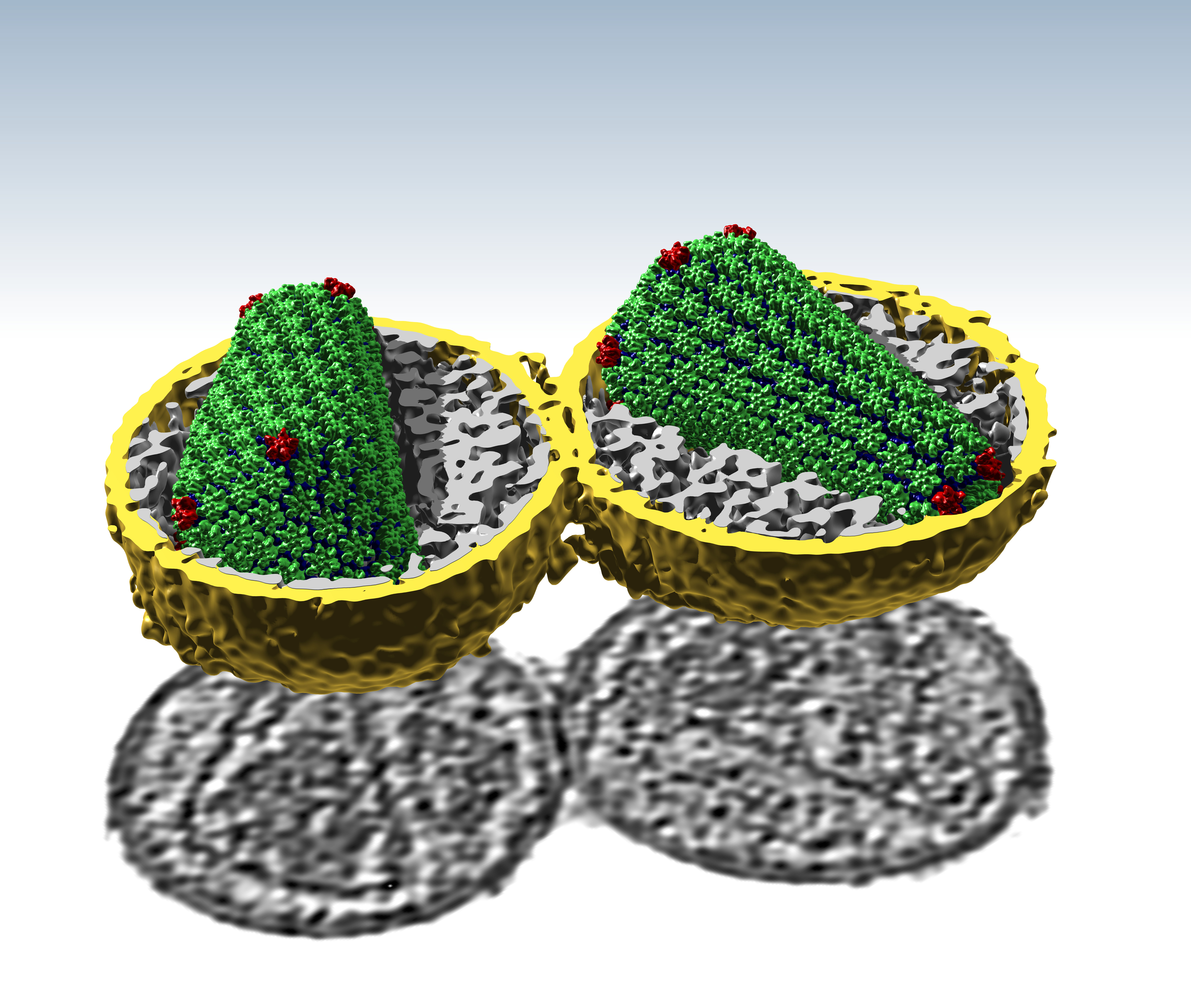
For the first time, the intricate structure of HIV capsid proteins has been visualised in the virus itself. Using subnanometre-resolution cryo-electron tomography, PhD student Simone Mattei looked inside individual HIV particles, showing just how the virus protects its genome.
In viruses, the viral genome is protected by a structure called a capsid, a shell made of proteins. For years, scientists across the world have probed and explored the unusual cone-shaped HIV capsid. Crystallographic studies combined with mathematical and computational models suggested how this shape was formed. Mattei’s work allows us to compare model and reality.
Cone shapes are quite unusual for protein assemblies and scientists wanted to know how the HIV capsid is structured. In the 1990s scientists first predicted that it had a fullerene structure (fullerene shapes are curved structures made from geometric shapes, such as a football made from hexagons and pentagons). Models suggested that hexamers and pentamers of protein create the curved surface of the conical HIV capsid. But no-one knew for sure what the capsid looked like within a real virus, or how the proteins bend and flex to form the cone. Now we do.
“Simone has been able to look at the virus itself and get structural data out of it. He can see both the structure of the proteins and how those subunits are arranged to make the cone,” explains EMBL group leader John Briggs. “In many cases that verified the crystallographic data. In other cases what we found was a bit different.”
For example, the hexamer structure is essentially as predicted, says Mattei who is part of the Molecular Medicine Partnership Unit group led by John Briggs (EMBL) and Hans-Georg Kräusslich (Heidelberg University Hospital). But the pentamer structure is different. And the flexibility of the interfaces between subunits (how much they vary around the structure) is also different from the models.
Each of Mattei’s images is a snapshot, a real intact virus that provides significantly more accurate data. This could help us understand how drugs act on the virus. and may also help identify new targets for drug development.


