Illuminating insulin release
EMBL researchers develop an optical method for measuring the release of insulin from single cells
Typical laboratory tests to measure levels of insulin – the hormone that controls our blood sugar – are based on the total amount of insulin secreted by a large number of cells. But exploring the fundamental biology behind this process – and testing possible drugs to control it – requires an understanding of how it works at the single-cell level. This is possible using a new method developed by researchers at EMBL and reported in Cell Chemical Biology, as study author Carsten Schultz explains.
What did you do?
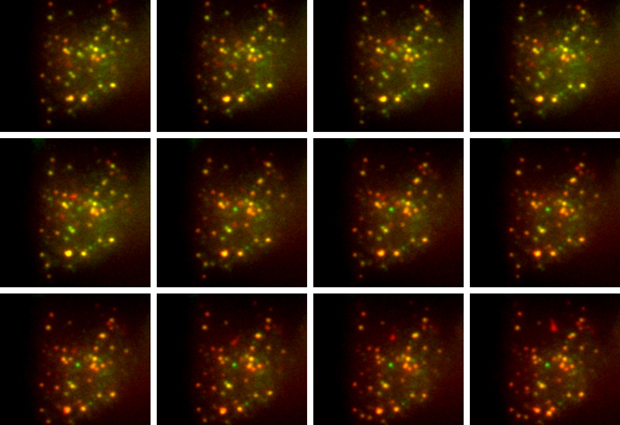
We used a virus to transfer a short DNA sequence into mouse beta cells – the specialised cells in the pancreas that release insulin. This DNA sequence caused the cell to produce a modified form of proinsulin, the molecule from which insulin is made. Proinsulin is a protein that consists of three parts: the A and B chains which together form insulin, and the C-peptide which gets separated from the other two during insulin formation. In our modified version, the cell produced proinsulin with a molecule of green fluorescent protein (GFP) attached to the A chain, and a molecule of a red fluorescent protein, called mCherry, attached to the C-peptide.
Our method could be useful in testing drug candidates for treating type 2 diabetes
Once insulin and the C-peptide are separated, a normal beta cell releases both molecules at the same time. Unexpectedly, in our modified system we found that only the GFP-tagged insulin was released, while the addition of mCherry appears to prevent the release of the C-peptide. As a result, there’s a reduction in the amount of green fluorescence in the cell as insulin is secreted, while the amount of red fluorescence remains constant. Taking the ratio of green to red fluorescence gives us a measure of the rate of insulin release.
Why does it matter?
This is a powerful method for measuring insulin secretion from single cells in real time. Having a ratiometric measurement – based on the ratio of green to red fluorescence – is particularly important when dealing with single cells, since it allows us to accurately compare measurements between cells. This is much more difficult if you only measure the absolute change in fluorescence intensity as insulin is released, since that doesn’t take account of natural cell-to-cell variability.
Our method is likely to be useful in observing the effects of drugs or drug candidates on the release of insulin, which is important in developing treatments for type 2 diabetes. Similar methods could also be used to explore the whole range of cellular processes that drive insulin secretion, which are still not fully understood.


