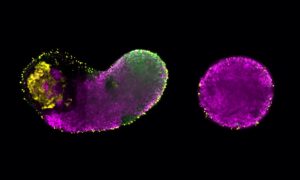
Cellular eavesdropping made easy
New method for identifying and measuring secreted proteins over time
In a nutshell:
- New method allows precise analysis of proteins released by cells over time (distinguishes them from proteins in the cells’ culture serum)
- Advantages: cells don’t have to be starved: avoids bias and allows more cell types to be studied; can follow fast reactions like immune response; applicable to cells taken directly from patients or other donors
- New approach for biomarker searches and drug screening
- First to unveil order of proteins released by macrophages in response to a bacterial component
It is much harder to keep up with a conversation in a crowded bar than in a quiet little café, but scientists wishing to eavesdrop on cells can now do so over the laboratory equivalent of a noisy room. A new method devised by scientists at the European Molecular Biology Laboratory (EMBL) in collaboration with the German Cancer Research Centre (DKFZ), both in Heidelberg, Germany, provides a new approach for studying the proteins cells release to communicate with each other, react to changes, or even to help them move. Published online today in Nature Biotechnology, the work also opens new avenues for drug and biomarker screening.
Cells in the lab have to be fed, and the ‘serum’ used to feed them contains proteins – many more proteins than the cells themselves secrete, or release into their environment. So for scientists attempting to eavesdrop on cells’ conversations, it’s like the cells are sitting in a room bustling with impenetrable chatter – until now. The new method developed by Jeroen Krijgsveld and colleagues allows scientists to distinguish proteins secreted by the cells from those in their food. And as they can measure exactly how much of each protein the cells have released, at just 2-hour intervals, scientists can see how secretion changes over time, for instance in response to changes in the cells’ environment.
The EMBL scientists coax cells into using an artificial amino acid instead of the methionine they would normally employ as one of the building blocks for their proteins. The researchers can then fish out the proteins released by the cells from the surrounding serum, using a technique called click chemistry. This does away with the need to starve cells, which was so far the most reliable way of being sure you were not ‘counting’ proteins from the serum. And this is an important development, as the new approach showed that starving cells, even just for a few hours, affects secretion.
The double advantage of not having to starve cells and being able to follow changes over time enabled Krijgsveld and colleagues to follow, for the first time, how white blood cells called macrophages – which can’t be grown without serum – react to a component of bacteria to kick off a rapid immune response.
“There’s much more for the community to explore,” Krijgsveld says: “our method could be used to watch how cells react to drug treatments; or to search for biomarkers, like the proteins cancer cells release that help them invade tissues; or to see how secretion changes if cells are grown in 3D instead of on a regular Petri dish. We’ve really seen a great deal of interest already.”
As well as continuing to investigate the intricacies of secretion, Krijgsveld’s lab now plan to use their new approach to study how cancer cells respond to drugs.
The work was done in collaboration with Stephan Herzig at DKFZ.
Further information:
Click chemistry technique developed at EMBL.
Article abstract
Secreted proteins constitute a large and biologically important subset of mammalian proteomes involved in cellular communication, adhesion and migration. Yet, secretomes are understudied because of technical limitations in the detection of low-abundant proteins against a background of serum used to sustain cell culture. Here we apply a novel method combining click-chemistry and pulsed SILAC labeling for the selective enrichment and quantification of secreted proteins irrespective of a complex protein background. We demonstrate its utility in the in-depth and differential analysis of secretomes, we show for the first time the effect of serum-starvation on secretome composition, and introduce a unique application studying the kinetics of protein secretion upon cellular stimulation.
Press Coverage
- SCIENCE OMEGA , 27 SEPTEMBER 2012Uncovering secretion secrets in cell cultures
- PROTEOMONITOR (GENOMEWEB) , 5 OCTOBER 2012EMBL Team Combines Click Chemistry and SILAC Mass Spec for Improved Secretome Analysis


