Fishing games gone wrong
Trial-and-error behind important cause of female infertility
When an egg cell is being formed, the cellular machinery which separates chromosomes is extremely imprecise at fishing them out of the cell’s interior, scientists at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, have discovered. The unexpected degree of trial-and-error involved in this process could explain why errors in the number of chromosomes in the egg cell are the leading cause of miscarriages and severe congenital diseases such as trisomies like Down’s syndrome, as well as an important cause of female infertility. The findings are published online today in Cell.
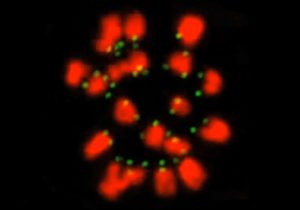
Our cells have two copies of each chromosome, one inherited from our mother and the other from our father. An oocyte, the cell that matures into an egg cell, has to discard half of its chromosomes, keeping only the maternal or paternal copy of each. To do so, fibres called microtubules act like fishing lines, attaching themselves to chromosomes and reeling them in to opposite sides of the cell. However, the EMBL scientists discovered that these microtubules are much worse fishermen than expected, often incorrectly hooking onto a chromosome and having to let it go again.
“We saw that they have to go through several tries before getting the connection right,” says Jan Ellenberg, who led the work at EMBL: “overall, 90% of all chromosomes get connected in the wrong way, and therefore the pathway that corrects these errors is heavily used.”
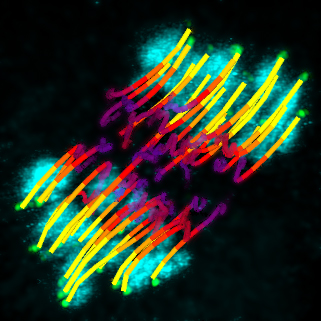
The difficulty in the oocyte is that two fishing lines cast from opposite sides of the cell have to attach themselves to the maternal and paternal copies of the same chromosome. Each of those chromosome copies has a protein structure called a kinetochore, which acts like the magnet in a toy fish, providing the spot for the microtubule ‘fishing lines’ to attach themselves. The EMBL scientists were the first to track the movement of all kinetochores throughout the whole 8 hours of the first round of cell division in mouse egg cells, which are very similar to human ones.
“We were able to get very high resolution images for extended periods of time,” explains Tomoya Kitajima, who carried out the work, “because our lab developed a microscope that automatically searches for chromosomes, zooms in, and scans only the area they are in, doing very little damage to the cell”.
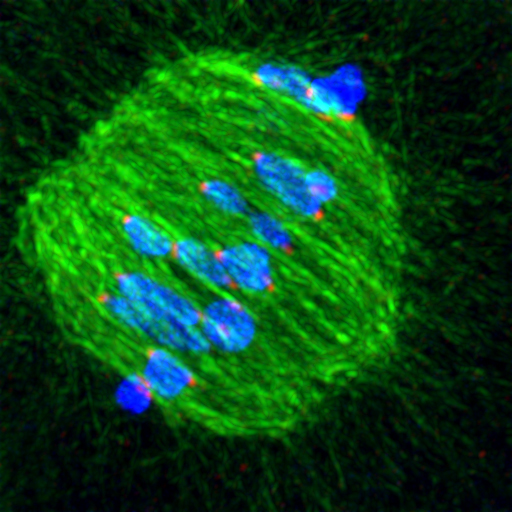
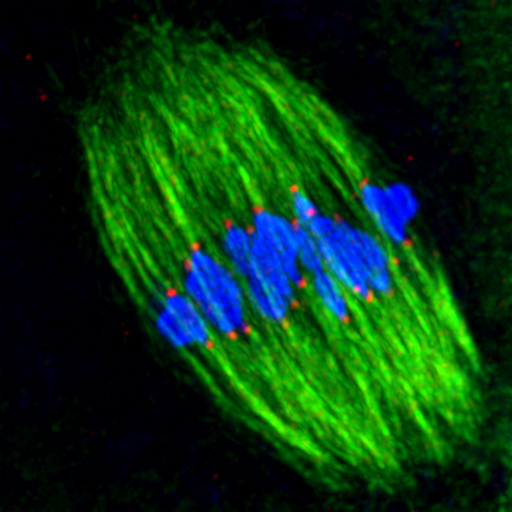
Children playing magnetic fishing games often accuse others of cheating, using their fishing rod to move a fish into a position that makes it easier to catch. Ellenberg and Kitajima’s time-lapse videos show that fishing microtubules also ‘cheat’ in this way. At earlier stages of cell division, before they start attaching themselves to kinetochores, microtubules interact with the arms of the chromosomes, nudging them into position in a ‘belt’ around the centre of the spindle.
But not even this chromosome belt, which had never been observed before, is enough to ensure that microtubules fish out the chromosomes correctly. The EMBL scientists’ results show that kinetochore attachment is much more error-prone in this type of cell division, called meiosis, than in mitosis, the simpler form of cell division through which other cells in our body split in two. This is probably because the egg cell precursor is an inordinately large cell, and because in meiosis microtubules emanate from around 80 different places in the cell, rather than stemming only from two poles as they do in mitosis.
“Our findings provide a very plausible explanation for the high rate of errors during egg formation. They form the basis to focus our future work on age-related female infertility, as it seems very likely that a component of the pathway that corrects these errors will be involved” Ellenberg concludes.