Futures: The dark proteome
As part of a series marking the tenth anniversary of the European Research Council, ERC grantee Edward Lemke shares his vision for the next ten years

The conventional view of proteins is that they have a well-defined three-dimensional structure, which makes them like perfectly optimised engines for carrying out a particular task. Determining a protein’s structure can help us understand more about its function and makes it easier to identify compounds that might modify its activity, which can be important in developing new drugs. However, about a third of proteins in the human body are part of what we call the dark proteome – a group of proteins whose structures cannot easily be determined, because they’re intrinsically disordered proteins (IDPs). IDPs don’t have a well-defined structure at all – they’re more like flexible strings. For a long time they couldn’t be studied because they don’t form crystals – as required for X-ray crystallography – and other techniques like electron microscopy don’t work well on flexible molecules.
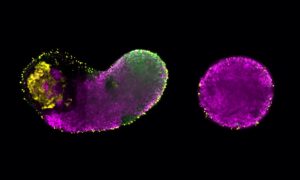
More recently, it’s been discovered that IDPs are involved in the formation of membraneless organelles – regions of a cell that perform certain functions and contain different chemicals to their surroundings, but without any membrane physically separating them from the rest of the cell. The way these organelles work is still mysterious, but they’re thought to play an important role in processes like cell division. Recent developments – including advanced fluorescence microscopy techniques – are making it possible to figure this out.
About a third of proteins in the human body are part of the dark proteome
One way IDPs can be studied is by modifying the DNA of a cell so that an IDP is expressed with a molecule of green fluorescent protein (GFP) attached to it. This molecule fluoresces when exposed to certain wavelengths of light, revealing the position of the IDP in the cell. Unfortunately GFP itself is quite large, so it prevents us from studying IDPs at the high resolution we would like to. We’re currently experimenting with alternative labelling strategies involving smaller semi-synthetic fluorescent molecules. In ten years I’d love to have a situation where no one would think of using GFP any more.
To truly understand the way IDPs function and how they interact with other molecules, we need to visualise them at a near atomistic level. We don’t have that kind of resolution yet – being able to do that is a ten-year vision or maybe even longer.

The European Research Council (ERC) is a European funding body that offers substantial five-year grants to support researchers at all stages of their careers. Inspired by the ERC’s tenth anniversary in 2017, we asked some of EMBL’s ERC grantees to look ahead another ten years and share their vision for their field of research.


