How drugs affect the life and death of proteins
Scientists at EMBL and Cellzome develop technology to monitor the effects of drug treatments on protein degradation and synthesis
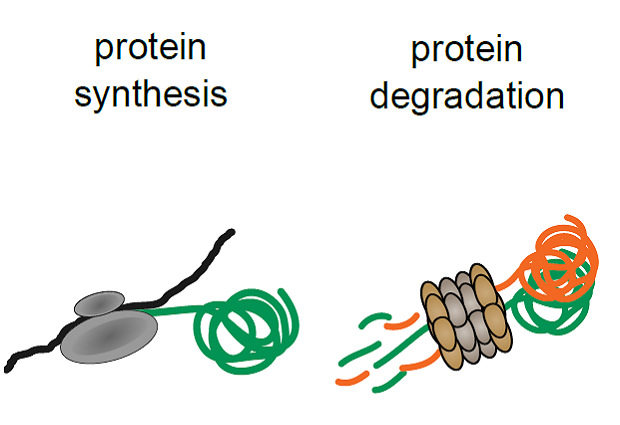
Proteins are responsible for countless activities in the cell, from directing their own production to transporting molecules across cell membranes. Protein levels are tightly controlled by balancing the rates at which they are broken down and replenished. Cells react to external stimuli, stress, or drug treatment by adjusting protein levels via orchestrating complex cellular mechanisms.
A lack of adequate tools has been hampering scientists’ ability to investigate protein degradation mechanisms, their role in disease, and their modulation by drug treatment. Now, scientists at EMBL and Cellzome have developed a technology to monitor the effects of drug treatments on protein degradation and synthesis. They publish their findings in Cell on 15 March.
What did you do?
We developed a technology that allows researchers to measure rates of protein degradation and synthesis following drug treatment or other stimuli, of thousands of proteins in a cell at the same time. With that, we can – for the first time – identify precise causes and effects of changes in protein homeostasis after protein-drug interactions, for example. The technology is called multiplexed proteome dynamics profiling, or mPDP.
To show the potential of mPDP, we performed three proof-of-concept studies. These demonstrate various mechanisms by which small molecule inhibitors can affect protein degradation. In one of these studies, we focused on heat shock protein 90 (HSP90): a chaperone associated with hundreds of other proteins in the human body, helping them fold into their correct shapes and acting to protect the cell from stress. HSP90 is also involved in many other cellular processes, ranging from DNA repair to the immune response, and is an attractive pharmaceutical target. By inhibiting HSP90 with a small molecule, we show that the proteins requiring HSP90 can be divided into two groups: they need HSP90 for folding purposes only – either to fold them to the correct shape when newly created or to re-fold them when damaged by stress – or they require HSP90 continuously during their entire lifetime. The latter group has a lower fold stability with changes in temperature, explaining why they might need HSP90 throughout their lifetime. Since HSP90 is an interesting pharmaceutical target, this distinction allows for more precise targeting of proteins in therapeutic strategies.
What are the possible implications of these results?
Protein degradation is related to diseases such as cancer or immunoinflammation, which can arise when the breakdown of a certain protein is altered. Our approach allows to discover how degradation of proteins is controlled after perturbations like drug treatment in health and disease. This opens new perspectives for the molecular understanding of pathological conditions, as well as for intervention strategies.
The researchers involved in this study are Mikhail Savitski and Isabelle Becher at EMBL, together with Nico Zinn, Maria Faelth-Savitski, Giovanna Bergamini, and Marcus Bantscheff, all at Cellzome, a GSK owned company.


