Hydraulic force shapes mammalian embryos
EMBL researchers uncover new role of fluid pressure in controlling embryo size and cell fate
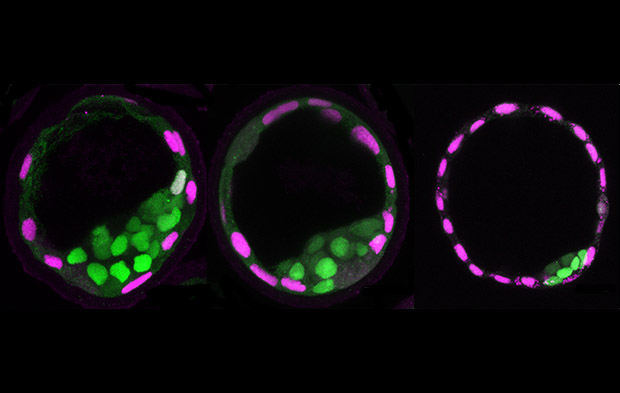

Fluid pressure inside an early mouse embryo plays an important role in controlling its size and determining the fate of its cells, according to researchers from EMBL and Harvard University. In their study, published in Nature, the team applied a new tool to directly measure the fluid pressure of a blastocoel (figure 1), which increases significantly during blastocyst maturation.
“We understand so little about the function of the blastocoel during the early development of an embryo,” says Chii Jou Chan, EMBL EIPOD and first author of the paper, “or how tissues sense and measure their size and control cellular behaviour accordingly.”
What is a blastocoel?
Following fertilisation, the mammalian egg cell begins to divide. At first, all of the newly formed cells are identical. However, during development, the cells have to differentiate into the large variety of cell types that carry out the many functions necessary for life.
One of the first points at which cells begin to differentiate is during the formation of the blastocyst, an early structure in the development of the mammalian embryo. The blastocyst consists of an inner cell mass that will go on to form the embryo itself, a surrounding layer of cells called thetrophectoderm that will become the placenta, and a cavity filled with fluid called the blastocoel.
Approximately one day after the blastocyst forms, it embeds itself into the uterine wall to continue with the next stages of development.
As the fluid pressure in the blastocoel increases, it causes increased mechanical stress in the surrounding trophectoderm cells. This triggers an active recruitment of junctional proteins to seal the junctions between adjacent cells (figure 2). This ‘mechanosensing’ mechanism allows the blastocyst to continue to grow.

However, when the cellular tension reaches a critical threshold, these proteins can no longer maintain adhesion between cells during cell division. Fluid begins to leak through the junctions in a process known as ‘blastocyst collapse’. This pressure decrease causes the junctions to seal again and the whole process is repeated. This causes the size of a mature blastocyst to oscillate about a certain point – a process accurately described by a theoretical model developed in collaboration with physicists from Harvard.
In the future, measuring blastocoel pressure with a tool like the one used here could be useful in monitoring the development of human embryos in in vitro fertilisation (IVF) clinics. This approach to measure fluid pressure in vivo also provides new opportunities to investigate the role of fluid pressure in organ development, such as in lungs or kidneys, or in other model systems such as sea animals or organoids.
In addition to controlling embryo size, this research also shows the effect that fluid pressure has on cell fate. Changes in fluid pressure and cavity size can influence the division pattern of the trophectoderm cells, and thereby affect their positions and which type of cell they become (see video below). These findings highlight the importance of considering hydraulic forces alongside the well-known indicators such as changes in gene expression patterns when studying cell fate decisions during embryonic development.
Fluorescent (left) and bright field (right) imaging of a blastocyst undergoing reduced expansion of its blastocoel. Reduced cavity size promotes events in which an outer cell (green nucleus with red dots) divides to generate an inner (yellow nucleus) and an outer (green nucleus) daughter cell. VIDEO: Chii Jou Chan/EMBL