Intruder detected: raise the alarm!
How a molecular switch activates the anti-viral innate immune response
When a thief breaks into a bank vault, sensors are activated and the alarm is raised. Cells have their own early-warning system for intruders, and scientists at the European Molecular Biology Laboratory (EMBL) in Grenoble, France, have discovered how a particular protein sounds that alarm when it detects invading viruses. The study, published today in Cell, is a key development in our understanding of the innate immune response, shedding light on how cells rapidly respond to a wide range of viruses including influenza, rabies and hepatitis.
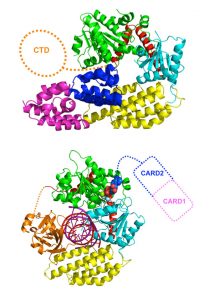
To sense invading agents, cells use proteins called pattern recognition receptors, which recognise and bind to molecular signatures carried only by the intruder. This binding causes the receptors to change shape, starting a chain-reaction that ultimately alerts the surrounding cells to the invasion. How these two processes – sensing and signalling – are connected, has until now remained unclear. The EMBL scientists have now discovered the precise structural mechanism by which one of these receptors, RIG-I, converts a change of shape into a signal.
“For a structural biologist this is a classic question: how does ligand binding to a receptor induce signalling?” says Stephen Cusack, who led the work. “We were particularly interested in answering it for RIG-I, as it targets practically all RNA viruses, including influenza, measles and hepatitis C.”
In response to a viral infection, RIG-I recognises viral genetic material – specifically, viral RNA – and primes the cell to produce the key anti-viral molecule, interferon. Interferon is secreted and picked up by surrounding cells, causing them to turn on hundreds of genes that act to combat the infection. To understand how RIG-I senses only viral RNA, and not the cell’s own RNA, and sounds the alarm, the scientists used intense X-ray beams generated at the European Synchrotron Radiation Facility (ESRF) to determine the three-dimensional atomic structure of RIG-I in the presence and absence of viral RNA, in a technique called X-ray crystallography. They found that in the absence of a viral infection, the receptor is ‘sleeping with one eye open’: the part of RIG-I that senses viral RNA is exposed, whilst the domains responsible for signalling are hidden, out of reach of the signalling machinery. When RIG-I detects viral RNA, it changes shape, ‘waking up’ the signalling domains, which become accessible to trigger interferon production. Although the EMBL scientists used RIG-I from the mallard duck, this receptor’s behaviour is identical to that of its human counterpart.
“RIG-I is activated in response to viral RNA, but a similar mechanism is likely to be used by a number of other immune receptors, whether they are specific to viruses or bacteria,” says PhD student Eva Kowalinski, who carried out most of the work.
Thus, these findings contribute to a broader understanding of the workings of the innate immune system – our first line of defence against intruders, and the subject of this year’s Nobel Prize in Physiology or Medicine. The work was carried out within the framework of the International Unit of Virus Host-Cell Interactions, a collaboration between EMBL, the University Joseph Fourier (UJF), in Grenoble, and the French Centre National de la Recherche Scientifique (CNRS) and also involved contributions from the laboratory of Denis Gerlier at the Institut National de la Santé et de la Recherche Médicale (INSERM), in Lyon, France.