Light-Seq: Light-directed in situ barcoding of biomolecules in fixed cells and tissues for spatially indexed sequencing
Nature Methods 10 October 2022
10.1038/s41592-022-01604-1
Researchers have combined advanced light microscopy with next-generation sequencing to create a method to study cells directly in the context of the tissues where they exist
Imagine looking at a section of biological tissue the way you view a city map on your smartphone. In this case, instead of zooming in on specific city landmarks for more information, you can zoom in on cells in tissues, having detailed molecular data at your fingertips.
Thanks to a new method called ‘Light-Seq’, what may seem impossibly futuristic may actually be possible in our future. Published in Nature Methods, researchers can now use this method to zero in on interesting cells under the microscope and study their gene expression in depth, leaving the cells intact for further study.
The power of fluorescence microscopy lies in its ability to identify and observe specific cell populations, e.g. ones with unique shapes, locations, or patterns of protein expression. On the other hand, single-cell transcriptomics technologies that help interpret the genome’s functional elements and that are driven by next-generation sequencing methods, give researchers unprecedented genome-level insights into all genes expressed within cells.
However, until recently, these two approaches remained largely segregated. We could view cells under the microscope, but only study the expression of a handful of genes at a time. Or conversely, we could characterise the transcriptomes of single cells, but since the process usually required the dissociation of the tissue into individual cells, we lost any information on cell location, shape, or other features. With the recent emergence of the spatial omics field, researchers are slowly gaining increasing access to these lost features.
Light-Seq – the new breakthrough technique that directly integrates imaging with sequencing of the same cells resulted from a collaboration between Sinem Saka at EMBL Heidelberg, Peng Yin’s group at the Wyss Institute for Biologically Inspired Engineering at Harvard University, and Constance Cepko’s team at the Blavatnik Institute at Harvard Medical School. It relies on a novel spatial transcriptomics workflow that combines fast light-induced DNA-crosslinking with a new type of DNA synthesis reaction.
In simple terms, researchers use light to attach small DNA sequences (called barcodes) to a cell’s transcriptional output. These barcodes later help identify the original position of these RNA sequences in a sample as researchers analyse transcriptomics data obtained through next-generation sequencing. Researchers can direct light to precise locations on a tissue section using laser scanning or by creating a “masking” effect using a device with small movable mirrors. This allows them to selectively barcode, or “geotag”, specific cells or regions of interest, and create barcoded sequencing libraries associated with them.
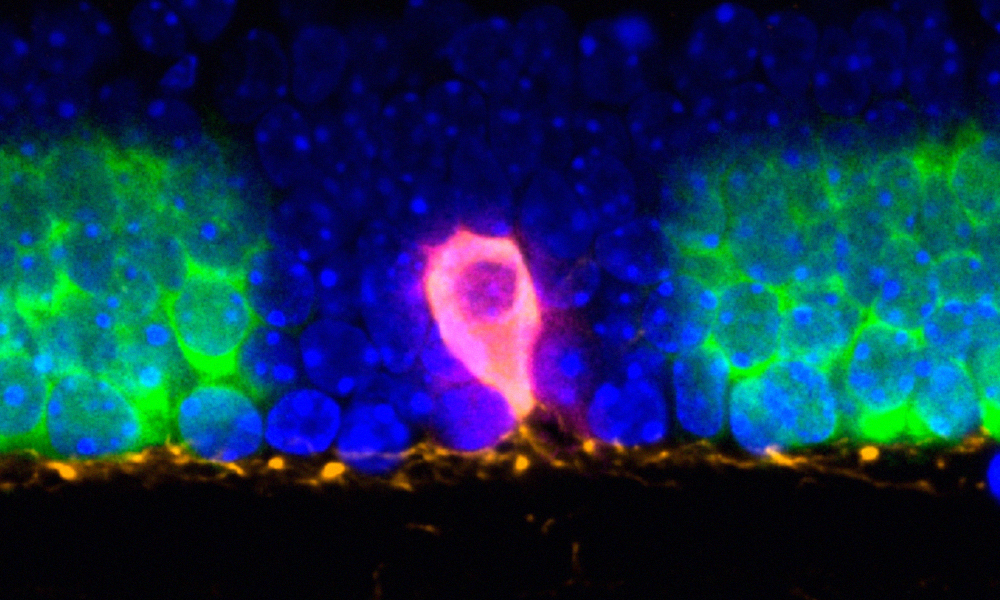
As a proof-of-concept, the scientists used Light-Seq to study dopaminergic amacrine cells, a very rare neuronal cell type. These cells form only 0.01% of the cells in the retina, and efficiently isolating them for in-depth analysis is extremely difficult. Using Light-Seq, the team could gather transcriptomics information from as few as four of these cells per tissue section and decipher the genes differentially expressed in this cell type compared to neighbouring cells. The researchers could also validate these newly identified biomarkers by further imaging experiments using an RNA detection method, SABER-FISH, that the team members previously developed.
Light-Seq is not only powerful in terms of its scope and flexibility, it is also inexpensive compared to other technologies since it uses components usually found in most laboratories. Unlike most other methods, it also leaves tissue sections intact, allowing researchers to continue studying them after collecting the transcriptomics data, to look at protein expression profiles, for example. Information gathered from such a framework could potentially provide important clues to understand phenotype-genotype associations involved in development and disease.
“There are many thousands of different RNA molecules per cell and they are just too densely packed to be captured in their entirety using present imaging techniques,” said co-first author Jocelyn Kishi, former Wyss Technology Development Fellow at Harvard University. “Light-Seq solves this problem by combining high-resolution barcode labelling with full-transcriptome sequencing, giving us the best of both worlds and additional key advantages.
Saka first began working on Light-Seq as a postdoc in Yin’s laboratory at Wyss Institute. “We conceptualised the project and designed the method before the pandemic and were able to validate a few of the parts individually,” she recalls. “But as we were getting close to putting everything together, the pandemic hit and it substantially changed our research priorities.”
While Saka and colleagues spent most of their precious allocated experimental time in the lab to help develop new COVID-19 diagnostics, they continued discussing the Light-Seq project. After months of downtime, often working at night, the data slowly began coming together.
Saka now continues to develop Light-Seq and its applications in her recently established lab at EMBL Heidelberg. “We are currently working on improving the method in terms of sensitivity, resolution, and multiplexing,” Saka said. “We are also starting to apply Light-Seq to study the transcriptomic variation at a subcellular level.”
Nature Methods 10 October 2022
10.1038/s41592-022-01604-1