Scratching the surface on cell differentiation
EMBL researchers develop a technique to reveal the important role of cell surface mechanical properties in determining how cells specialise
Over the past twenty years, an increasing body of research has focused on analysing the mechanical forces that help regulate how cells change and develop. Now, EMBL scientists have demonstrated the importance of physical forces in cell differentiation. This is the process by which a cell changes from one type to another – usually more specialised – type of cell. The results are published in the journal Cell Stem Cell.
As cells differentiate and take on specialised functions, they also change their shape. Scientists know that cell shape is governed by the mechanics of the cell surface, but until now research has only scratched the surface of understanding if these mechanics can also control cell differentiation.
Scientists in the Diz-Muñoz group at EMBL Heidelberg are working to build understanding of the role that mechanical properties play in affecting cell behaviour – a young and rapidly developing field of study. They have developed and successfully used a highly specialised technique to manipulate the mechanical properties of the membrane at the cell surface, enabling them to analyse membrane mechanics at a level of detail that few other laboratories can achieve.
Stem cell differentiation
Stem cells are the cells from which other, specialised cells originate. Their unique ability to become specialised cells with a distinct function – such as brain or heart cells – means they’re incredibly important for our understanding of development, and they play a crucial role in biomedical research.
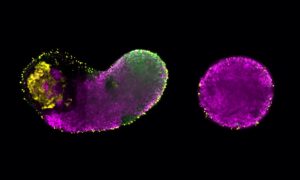
Working with stem cells derived from a mouse embryo, members of the Diz-Muñoz group were able to show for the first time that the mechanical properties of the cell membrane co-regulate the process of stem cell differentiation and specialisation, alongside the cell’s genome.
“This is something completely new, and will need to be taken into account for future biomedical research,” says Sergio Lembo, one of the authors of the study. “Take something like creating organoids, which are simplified 3D versions of organs. They provide model systems for scientists to study diseases, and are made using stem cells. Being able to master cell differentiation is essential for the creation of organoids, and our work has shown for the first time how important the mechanical properties of the cell membrane are in that process.”
A groundbreaking tool
The surface of animal cells can be imagined as a balloon with another balloon inside it. The outer surface is called the plasma membrane, and is made primarily of fat molecules. The inner surface, the cortex, is a mesh of a threadlike protein called actin. These two layers are glued together by sticky proteins, and this glue is known as the membrane-to-cortex attachment.
The Diz-Muñoz group engineered a specific molecule that enabled them to tune the membrane-to-cortex attachment – altering its strength to specific degrees. This molecule acts as an artificial glue, allowing the researchers to make tiny alterations to the levels of adhesion between the plasma membrane and the cortex. The result is an unparalleled tool to investigate the role of cell surface mechanics. Thanks to the use of this molecule, the Diz-Muñoz group showed that stem cells need to reduce membrane-to-cortex attachment to differentiate and hence specialise. This is consistent with the idea that cells acquire different shapes during differentiation, since keeping a high adhesion between the cell membrane and the cortex makes the cell surface resistant to being moulded into different forms.
Next steps
The researchers hope that a deeper understanding of cell surface mechanics will bring new opportunities for controlling stem cell differentiation. In the longer term, the ability to control this process could play an important role in treating disease or tissue damage, through approaches such as regenerative medicine, which is based on the ability of stem cells to repair damaged tissues.


