Arrested coalescence of multicellular aggregates
Soft Matter 5th May 2022
10.1039/D2SM00063F
EMBL researchers revise the old problem of sintering droplets to understand the mechanical properties of tissues
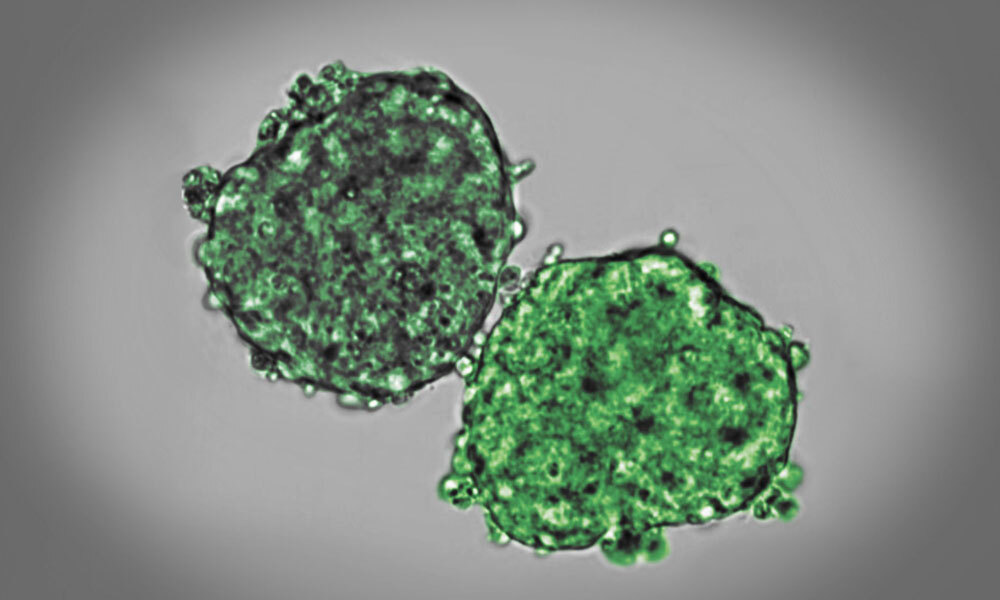
When an embryo develops, its cells divide, move, and interact. In doing so, the mechanical properties of tissues, such as viscosity and elasticity, change over time. David Oriola, a biophysicist in the Trivedi Group, studies the physical changes that tissues undergo during early development. He combines experiments with mathematical modelling to explain how cells self-organize to form tissues. His most recent study was now published in Soft Matter.
“Shortly before the lockdown started, I was studying the fusion of embryonic stem cell aggregates. To my surprise, the two aggregates only fused partially,” said Oriola. “I later found out this process is known as arrested coalescence, and it is a common phenomenon in soft matter physics.”
Arrested coalescence is very important in the manufacturing of petroleum, beauty products and certain processed foods. For example, in the food industry, the consistency and texture of whipped cream depends on the partial coalescence of cream’s fat droplets.
The existing physical models describing the fusion of cellular aggregates did not contemplate the possibility of partial coalescence, and therefore could not account for Oriola’s experimental findings. However, before he could repeat the experiments, the pandemic arrived and the lab went into lockdown.
“I started to review the literature on the physics of droplet sintering. The first mathematical descriptions of sintering go all the way back to 1945, when Frenkel was trying to explain the sintering of metal droplets,” explained Oriola. “Since we were all at home, not being able to do new experiments, I reproduced the calculations and re-derived the equations of viscous sintering.”
Oriola realized that the mathematical models used to study the fusion of cellular aggregates considered tissues as purely viscous materials. However, such models could not account for arrested coalescence because they do not consider elasticity that is characteristic of viscoelastic materials like biological tissues.
“I revisited the mathematical models and I decided to model tissues as viscoelastic solids, instead of viscous fluids, which means that elastic effects persist even at long timescales. The next step was to see if this model could match the experimental data,” said Oriola.
And it did. The model successfully explained the fusion dynamics of the cellular aggregates as well as how the fusion dynamics change with the aggregate size. In this way, this model provides a fast and inexpensive method to study the mechanical properties of tissues from simple time-lapse fusion events. In addition, together with the Ebisuya Group, the researchers showed that the model successfully explained the fusion of other types of stem cell aggregates. Finally, in collaboration with the Sharpe Group, the fusion experiments were reproduced using computational simulations that helped them to understand how the microscopic cellular properties relate to the macroscopic material properties.
The work of David Oriola and colleagues is very much in line with EMBL’s New Programme, and more concretely with the research theme Theory@EMBL, which promotes theory-guided paths to understanding the underlying principles of biological systems.
“What is encouraging about this model is that it could have wider implications beyond biology. In principle, the same method could be applied to characterise the material properties of microemulsions just by looking at how fat globules fuse,” said Oriola.
Soft Matter 5th May 2022
10.1039/D2SM00063F