Without a trace
Cells keep to one direction by erasing the path
In a nutshell:
– Zebrafish embryo’s cells can move in one direction by creating their own gradient
– Could have implications for cancer and metastasis
– Discovered using a tag that changes colour as its target ages
Migrating cells, it seems, cover their tracks not for fear of being followed, but to keep moving forward. Scientists at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, have now shown that cells in a zebrafish embryo determine which direction they move in by effectively erasing the path behind them. The findings, published online today in Nature, could have implications not just for development but also for cancer and metastasis.
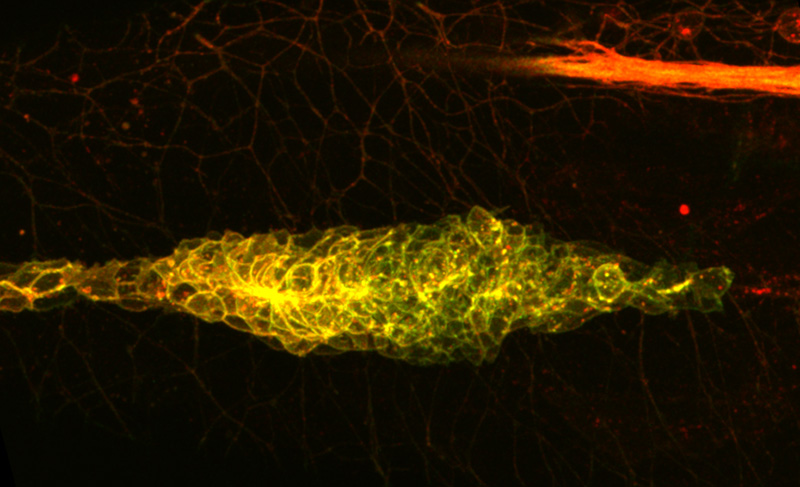
As a zebrafish embryo develops, a group of cells migrate down the side of its body, leaving clumps of cells along the way. Those clumps will become ear-like organs, sensing vibrations in the water. In the adult fish, this is called the lateral line, so the moving mass in the embryo is dubbed the lateral line primordium. To migrate, these cells follow a trail of a molecule called a chemokine – but how do they know to keep moving in the same direction? Scientists assumed that the trail was a one-way path: a gradient where cells moved from less- to more-concentrated chemokine. But Darren Gilmour and colleagues at EMBL have now found that, rather than being produced outside them, that gradient is actually generated by the cells themselves.
“We found that the cells at the rear of the group have a ‘vacuum cleaner’,” says Erika Donà, who carried out the work. “They suck up the chemokine at the back, but at the front there’s still a lot of chemokine to follow, so the cells move forward.”
To investigate the role of the ‘vacuum cleaner’ molecule, Gilmour and Donà turned to a ‘detector’ molecule which all cells in the primordium use to sniff out the chemokine, and which the scientists labelled with a tag that goes from green to red as the detector ages.
Video 1: Zebrafish lateral line migration: painting by age
Cells at the front of the primordium glowed green, showing they were in such frequent contact with the chemokine that their detectors were constantly being renewed, while cells at the rear encountered so little chemokine that their detectors had a chance to grow old, painting the cells red. To show that this gradient is created by the act of sucking up the chemokine, the scientists genetically engineered fish to have the vacuum cleaner molecule in an accompanying nerve rather than at the rear of the primordium itself. When the vacuum cleaner was switched to the nerve, the nerve went from following the migrating primordium to guiding it.
Video 2: Zebrafish lateral line migration: One direction
“It makes a lot of sense for the cells to choose their own direction,” says Darren Gilmour, who led the work. “There’s a lot going on in the embryo, lots of cells moving in lots of directions, so it may be very difficult to sustain a gradient. What we’ve shown is that you don’t always need to.”
The study could also be relevant to another, seemingly very different type of moving cells: those in metastasising cancers. Scientists have found that both the ‘detector’ and the ‘vacuum cleaner’ molecule play important roles in different tumours’ ability to metastasise – to spread from one place to another in the body. These findings hint at what those roles might be, and consequently at possible ways to block them.
The colour-changing tag used in this study was first developed by Anton Khmelinskii in the group of EMBL Alumnus Michael Knop, now at the DKFZ-ZMBH Alliance. Joseph Barry in the Huber group helped develop and apply data analysis methods for this work.
Further information
Simulations by the Hufnagel group showed that, in principle, ‘vacuuming’ could direct movement
How the lateral line primordium moves – previous work by the Gilmour lab
Zebrafish and other model organisms used at EMBL (podcast and image gallery)
More videos of lateral line formation by the Gilmour lab: ‘Becoming a leader’; ‘Migration makes (a) sense’



